Effects on Joints and Arthritis
|
Until recently it seems that most joints were thought to be sterile. Partly, this happened because it is so difficult to culture many bacteria. But since the advent of PCR testing, it’s pretty clear that many of the oral pathogens CAN get into joint spaces and may well be doing damage there. And getting there is half the problem. How the body responds is probably of equal import. Hyper-reactors may well show more damage once the bacteria or their wasteproducts appear in the joint spaces. |
Is there a relationship between rheumatoid arthritis and periodontal disease?
F.Mercado 1 , Roderick I.Marshall 1 , Alexander C.Klestov 2 and P. MarkBartold 1
Journal of Clinical Periodontology Volume 27 Issue 4, Pages 267 – 272 Published Online: 24 Dec 2001
Aim: The aim of this study was to determine whether there is a relationship between disease experience of rheumatoid arthritis and periodontal disease.
Methods: 1412 individuals attending the University of Queensland’s School of Dentistry were assessed for the prevalence of periodontal disease and rheumatoid arthritis. Analysis of data obtained from a self-reported health questionnaire and dental records was carried out and included: number of individuals referred for advanced periodontal care (test group); number of individuals attending for routine dentistry; determination of rheumatoid arthritis, cardiovascular disease and diabetes mellitus through self-reporting and assessment of prescription medications; assessment of periodontal disease through assessment of existing oral radiographs.
Results: In patients referred for periodontal treatment, the prevalence of self-reported rheumatoid arthritis was 3.95% which is significantly higher than that seen in patients not referred for periodontal treatment (0.66%) and also that reported in the general population (1%). Of those referred patients with rheumatoid arthritis, 62.5% had advanced forms of periodontal disease. These results were mirrored in the results of the self-reported prevalence of cardiovascular disease and diabetes mellitus which was consistent with the published higher prevalence in periodontal patients.
Conclusions: Based on data derived from self-reported health conditions, and not withstanding the limitations of such a study, we conclude that there is good evidence to suggest that individuals with moderate to severe periodontal disease are at higher risk of suffering from rheumatoid arthritis and vice versa.
| Abstract Journal of Periodontology 2006, Vol. 77, No. 2, Pages 280-288 (doi:10.1902/jop.2006.050051) Periodontal and Hematological Characteristics Associated With Aggressive Periodontitis, Juvenile Idiopathic Arthritis, and Rheumatoid Arthritis Anne Havemose-Poulsen,* Jytte Westergaard,* Kaj Stoltze,* Henrik Skjødt, Background: Periodontitis shares several clinical and pathogenic characteristics with chronic arthritis, and there is some degree of coexistence. The aims of this study were to elucidate whether patients with localized aggressive periodontitis (LAgP), generalized aggressive periodontitis (GAgP), juvenile idiopathic arthritis (JIA), and rheumatoid arthritis (RA) share periodontal and hematological characteristics distinguishing them from individuals free of diseases. Methods: The study population consisted of white adults (≤35 years old) with LAgP (N = 18), GAgP (N = 27), JIA (N = 10), RA (N = 23), and healthy controls (N = 25). All individuals underwent a standardized interview, blood sampling, and an intraoral examination, including registration of plaque, bleeding on probing, probing depth (PD), clinical attachment loss (CAL), and alveolar bone loss (ABL) on radiographs. Blood samples were analyzed for erythrocyte fraction, leukocytes and differential counts, erythrocyte sedimentation rate, C-reactive protein (CRP), immunoglobulin (Ig) M and IgA rheumatoid factors (RFs), and antibodies to cyclic citrullinated peptides. Results: RA patients had a higher percentage of sites with PD ≥4 mm, CAL ≥2 mm, and ABL ≥2 mm compared to controls. The percentage of sites with CAL ≥2 mm significantly correlated with the levels of IgM-RF and IgA-RF. Missing teeth in JIA and RA patients were not lost due to periodontitis. Patients with GAgP showed higher levels of leukocytes, including neutrophils, and CRP compared to controls. In part, JIA and RA patients showed similar results. Conclusions: Young adults with RA may develop periodontal destruction, and these patients require professional attention. Both differences and similarities in periodontal and hematological variables were seen in individuals with periodontitis, JIA, and RA. |
Clin Exp Rheumatol. 2002 Jul-Aug;20(4):555-7.
16-year remission of rheumatoid arthritis after unusually vigorous treatment of closed dental foci.
Breebaart AC, Bijlsma JW, van Eden W.
Department of Ophthalmology, University of Amsterdam, The Netherlands. [email protected]
Abstract
This report describes a remission of rheumatoid arthritis (RA) of 16 years duration, apparently caused by the extraction of endodontically well-treated, healthy looking teeth. The only clue that the teeth were contributing to the disease pathogenesis in this case of RA was that the patient was able to reproducibly induce severe attacks of arthritis after prolonged, heavy pressure on some of his teeth treated with root canal fillings. After extraction, a small pus layer was found to cover the apices of the clinically healthy looking teeth. The rheumatoid factor (RF) became negative and the patient remained symptom free for the next 16 years. The possible connections between micro-organisms in closed dental foci under constant pressure and the chronicity and exacerbations of RA are discussed.
| http://www.medscape.com/viewarticle/505458 Original Article
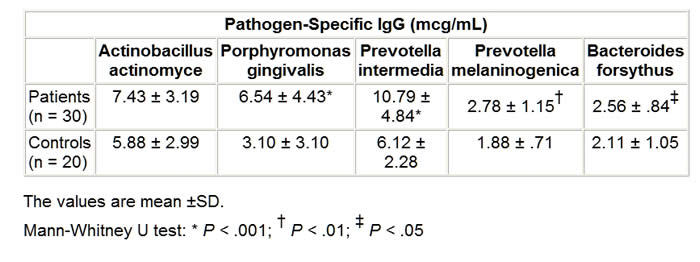
Serum Antibodies to Oral Anaerobic Bacteria in Patients With Rheumatoid Arthritis Abstract and IntroductionAbstractBackground: This study was conducted to determine the component that causes the disease in rheumatoid arthritis (RA), which shows great resemblance to periodontitis in a pathologic context. IntroductionRheumatoid arthritis (RA) is a polyarticular, chronic, inflammatory, and systemic disease.[1] In many previous studies, this rheumatic disease was found at high ratios for individuals with periodontitis and RA shows resemblance to periodontitis in many aspects pathologically.[2,3] HLA-DR4 tissue antigens are found at high frequencies in both patients with periodontitis and with RA. HLA-DR4 tissue antigens and their subtypes are directly associated with each disease.[4,5] Porphyromonas gingivalis, Prevotella intermedia, Prevotella melaninogenica , and Bacteroides forsythus are gram-negative small basil quality obligate anaerobic bacteria and are held directly responsible for the formation of periodontitis (Periodontopathic bacteria). These bacteria usually secrete brown-black pigments and form colonies when they reproduce in blood agar plates used for their cultivation.[6] These bacteria were classified in the Bacteroides genus until 1988 and 1990, when they were reclassified to the Porphyromonas and Prevotella genera, respectively, in accordance with new classification strategies made by Shah and Collins.[7,8] These bacteria are members of the normal human mouth flora, where they cause endodontitis, odontogenic inflammation, gingivitis, and mainly periodontitis. They are also found commensally in the body flora, where they cause chronic sinusitis, chronic recurrent tonsillitis, bronchitis, pneumonia, chronic otitis media, parotitis, intra-abdominal infection, genitourinary infection, and wound infections in immune-suppressed individuals as well as when in conjunction with facultative anaerobic bacteria (ie, Streptococcus pyogenes, Staphylococcus aureus, Haemophilus influenzae, Klebsiella pneumonia, Proteus mirabilis, and Escherichia coli ).[9] A significant point here is that, although these bacteria are obligate anaerobic bacteria, when in conjunction with the facultative anaerobic bacteria mentioned above, they lead to mixed types of infections. In this study, we investigated the oral Bacteroides, Porphyromonas, and Prevotella bacteria antibodies usually found in periodontitis etiopathogenesis but from serum samples of RA patients. Materials and MethodsPatients and ControlsThis study was conducted from August 2001 to August 2002 in Turkey and Australia. The study was conducted in accordance with the principles of Good Clinical Practice, according to the Declaration of Helsinki. Before this study, all patients gave written informed consent. Thirty patients (5 men, 25 women) who fulfilled the American College of Rheumatology criteria for RA were included.[10] The mean age of RA patients was 49 years with a range of 19-69. The mean disease duration was 4.9 ± 1.3 years. Patients were ineligible to participate in the study if they met any of the following exclusion criteria: Sjögren’s syndrome, other infectious disease, metabolic disease, periodontal disease, gingivitis. treatment with antibiotics, and using tobacco. Rheumatoid factor (RF) was measured by agglutination assay (latex) test in the 30 patients with RA. For each patient, the disease activity score (DAS28) was also calculated from the number of tender and swollen joints (both by 28-joint-count), erythrocyte sedimentation rate (ESR), and patient’s general health assessment by visual analog scale. The presence of extra-articular manifestations, such as Sjögren’s syndrome, rheumatoid nodules, rheumatoid vasculitis, pleuritis, nephropathy, anemia, Raynaud’s syndrome, or Felty’s syndrome, was recorded and vasculitis was diagnosed when one of the following symptoms was present: polyneuropathy/mononeuritis multiplex, cutaneous vasculitis, digital gangrene, and visceral infarction, not attributable to any disease. The control group consisted of blood serum samples obtained from 20 (5 men, 15 women) healthy donors. The mean age of healthy donors was 47 years with a range of 23-69. There was no clinical evidence of periodontitis and gingivitis in any of the controls. The serum samples were sent to the University of Queensland School of Dentistry (Oral Biology and Pathology Laboratory) in Brisbane, Australia, for the bacteria antibody determination and quantification. Cultivation of BacteriaBacteria were revived from liquid nitrogen stocks and P gingivalis , Actinobacillus actinomycetemcomitans , and P intermedia were grown on a Trypticase Soy Agar (TSA). This agar was prepared from at Trypticase soy broth base (30 g/L; BBL, Becton Dickinson, Cockeyville, Maryland) with the addition of agar (10 g/L), yeast extract (5 g/L), L-cysteine hydrochloride (.5 g/L), sodium formate (2 g/L), sodium fumerate (3 g/L), menadione (1 mg/L), haemin (5 mg/L), L-cysteine HCl (.5 g/L), and 5% defibrinated horse blood. P melaninogenica was grown on Wilken Chalgrens sheep blood agar plates. Tannerella forsythensis was grown on TSA with a disk containing 300 mcg of N -acetylmuramic acid (NAM) and co-cultured with streak S aureus . Plates were incubated at 37°C in atmosphere of 80% N2, 10% CO2, and 10% H2 in anaerobic jars for 4-5 days. Purity was monitored by Gram stain, colonial morphology on blood agar plates, and identification confirmed using API-ZYM. The strains were expanded in 550-mL batch cultures of brain heart infusion broth (Difco Laboratories, Detroit, Michigan), supplemented with yeast extract (5 g/L), haemin (5 mg/L), and menadione (1 mg/L), L-cysteine HCl (1 g/L), and for the cultivation of B forsythus , NAM (15 mg/L) was added to the broth medium. Bacteria were incubated at 37°C in an atmosphere 80% N2, 10% CO2, 10% H2 for 48-72 hours. P melaninogenica was harvested from Wilken Chalgrens sheep blood agar plates. Purity was assessed by Gram stain and colonial morphology on agar plates. Bacteria were harvested at late log phase by centrifugation (10,000 x g for 20 minutes) and washed twice in .15 M sodium chloride. Bacteria were revived from liquid nitrogen stocks and grown on a TSA su . B forsythus was prepared from a trypticase soy broth base (30 g/L; BBL, Becton Dickinson, Cockeyville) with the addition of agar (10 g/L), yeast extract (5 g/L), L-cysteine hydrochloride (0.5 g/L), menadione (1 mg/L), haemin (5 mg/L), and 5% defibrinated horse blood. B forsythus was grown on TSA with a disk containing 300 mcg of NAM and co-cultured with streak of S aureus . Plates were incubated for 7-10 days at 37°C in an atmosphere of 80% N2, 10% CO2, and 10% H2 in anaerobic jars. Purity was monitored by Gram stain, colonial morphology on blood agar plates, and identification confirmed using API-ZYM. The bacteria was expanded in a 500-mL batch culture of brain heart infusion broth (Difco Laboratories, Detroit), supplemented with yeast extract (5 g/L), haemin (5 mg/L), menadione (1 mg/L), and L-cysteine HCL (1 g/L), and NAM (15 mg/L). Broth cultures were incubated at 37°C in an atmosphere of 80% N2, 10% CO2, and 10% H2 for 48-72 hours. Purity was assessed by Gram stain and colonial morphology on TSA. Bacteria were harvested at late log phase by centrifugation (10,000 x g for min) and washed twice in .15 M sodium chloride. Serum Antibody AssayPathogen-specific immunoglobulin (Ig)G was quantitated with an ELISA. Microtiter plate wells (Nunc, Maxisorp) were coated with 100-mcL aliquots of bacteria in .1 M carbonate buffer (pH 9.6) at a concentration of 1 mcg/mL. To allow quantification of antibody titer, 100-mcL aliquots of known concentrations of IgG prepared from purified human IgG (Zymed) was also transferred to the plate. Negative control wells for each serum sample were prepared by adding carbonate buffer only to the appropriate wells. After overnight incubation at 4°C, the plates were washed (x 3) in PBS-Tween and blocked with 1% BSA for 1 hour at room temperature. One hundred-microliter aliquots of diluted serum were then added to the appropriate wells, and the plates incubated at room temperature for 2 hours. After a further wash (x 3) in PBS-T, plates were incubated for 1 hour at room temperature with diluted rabbit antihuman IgG-specific horseradish peroxidase-labeled monoclonal antibody (Dako). After a further wash (x 3) in PBS-T, color development was achieved by adding 150 mcL of 2.5 mM tolidine (Eastman Kodak, Rochester, New York) in 100 mM phosphate citrate buffer pH 3.5 containing .025 mM ethylenediaminetetraacetic acid and activated with 3% H2O2. The resulting reaction was stopped after 10 minutes by adding 50 mcL of 1 M HCl. Plates were then read in a Bio-Rad microplate reader, model 3550 (Bio-Rad Laboratories, Regents Park NSW, Australia ) at 450 nm and 655 nm. Statistical AnalysisMann-Whitney U test was used to compare patients with controls. The results were expressed as mean values plus/minus standard deviation (SD), and statistical significance was defined as P < .05. The association between the antibody levels to oral anaerobic bacteria and acute-phase reactants (ESR, C-reactive protein [CRP]) was analyzed using the correlation coefficient ( r ). Also, the association between the antibody levels to oral anaerobic bacteria and disease duration and age of patients was analyzed using the correlation coefficient ( r ). ResultsThe results are shown in Table 1 . The RA patients had significantly elevated IgG antibodies to P gingivalis ( P < .001), P intermedia ( P < .001), P melaninogenica ( P < .01), and B forsythus ( P < .05) when compared with healthy controls. The antibody levels to A actinomycetemcomitans ( P > .05) did not display any significant difference between the 2 levels. The mean ESR was 52.8 mm/h (SD ± 13.8), and serum CRP was 32.0 mg/L (±10.6) in patients with RA. The mean DAS28 score was 4.2 ± 1.3 in RA patients. RF was strongly positive in serum samples obtained from all patients with RA. The median morning stiffness duration of all patients was 60 minutes. Extra-articular manifestations (rheumatoid nodules and anemia) have been demonstrated in 6 of 30 RA patients (20%). Vasculitis has been demonstrated in 1 of 30 RA patients (3.3%). A positive correlation between serum IgG antibody levels against P gingivalis and serum CRP was clearly detected in patients with RA ( r = .795; P = .000; Figure 1). There was significant correlation between serum IgG antibody levels against P intermedia and ESR ( r = .809; P = .000) (Figure 2). In RA patients, there was no significant correlation between IgG antibody levels against P melaninogenica,B forsythus, and the acute-phase reactants ESR or serum CRP ( P melaninogenica IgG: ESR: r = .019, not significant [NS], CRP: r = -.141, NS; B forsythus IgG: ESR: r = .173, NS, CRP: r = .147, NS; data not shown).
Poryphyromonas gingivalis and serum C-reactive protein detected in patients with rheumatoid arthritis.
Correlation between serum immunoglobulin (Ig)G antibody levels against Prevotella intermedia and erythrocyte sedimentation rate. There was no significant correlation between IgG antibody levels against oral anaerobic bacteria and disease duration and age of patients ( P gingivalis IgG: disease duration: r = +.157, NS, age: r = +.119, NS ; P intermedia IgG: disease duration: r = -.132, NS, age: r = +.166, NS; P melaninogenica IgG: disease duration: r = +.031, NS, age: r = +.112, NS; B forsythus IgG: disease duration: r = +.023, NS, age: r = +.127, NS). DiscussionP gingivalis has arginine- and lysine-specific proteinases and T forsythensis (formerly B forsythus ) has arginine-specific proteinase.[11] Stastny[12] stated the relationship between the RA and HLA-DR4 in 1978. If we consider that RA-related HLA DR molecules bind with 1 of the P gingivalis or B forsythus virulence factors, when the bacteria is linked to the amino acid codon in the third hypervariable region, then the arginine (R) and lysine (K) amino acids will be selectively and specifically denatured by arginine-specific and lysine-specific proteinase and the different codon will be observed. This codon will be considered alien by the organism, and an immune response will be generated against it, or a T-cell response will be generated against all appropriate molecules presented by this major histocompatibility complex class II molecule. RFs have been identified as autoantibodies that react to the IgG molecule in the Fc region, and these antibodies may be of the IgM, A, G, or E epitopes.[13] P gingivalis proteinase is responsible for the epitope development in the RF Fc region. Bonagura and colleagues identified the lysine, histidine, arginine amino acid sequences for the Fc region of the IgG molecule.[14] Because P gingivalis specifically decomposes lysine and arginine; the IgG3 CH2 and CH3 domains processed by P gingivalis proteinase become powerful targets for the RF produced by rheumatoid cells.[15] In patients with RA, loss of galactose residues on IgG has been observed as a glucosylation defect of Fc and this finding leads to a worse prognosis in patients who have agalactosyl IgG.[13] P melaninogenica as a saccharolytic bacteria disintegrates galactose. Consequently, P melaninogenica infection in these patients who have agalactosyl causes this condition by binding to the Fc region of the IgG molecule and metabolizing galactose with its enzymes. Gioannini and colleagues[16] investigated the expression of the interleukin-8 (IL-8) inherent in the human umbilical vein endothelial cell (HUVEC) by using as agents the lipopolysaccharide binding protein (LBP) and soluble CD14 (sCD14) of the lipooligosaccharides (LOS) obtained fromNeisseria meningitidis serotype B. According to this study, LOS:sCD14 complex does not require LBP to activate HUVEC. P gingivalis lipopolysaccharides show lower affinity to LBP than E coli lipopolysaccharides.[17] Lactoferrin is a lipid-a binding protein, and P gingivalis degrades it.[18,19] P gingivalis also degrades albumin and hemalbumin.[20] Thus it may be argued that the real molecule that acts as an agent for the transport of P gingivalis endotoxin is the sCD14. The 57- to 64-amino acid range in the sCD14 molecules are specific to the lipopolysaccharide binding.[21] In a study performed by McGinley and associates in 1995, it was shown that the 57- to 65-amino acid range of sCD14 protects itself from enzyme disintegration using Asp-N as an endoprotease.[22] Also, a series of alanine substitution mutants of the sCD14 molecule were obtained. Therefore, the principal antigenic peptide presented to T cells by antigen-presenting cells is the amino acid sequence 57-64 within the sCD14 molecule. This is probably the same principal peptide presented in RA. Some T-cell receptor (TCR) Vb genes are present more frequently in patients with RA than in control subjects.[23-25] Similarly, Leung and Torres showed that P intermedia specifically stimulates the expression of Vbeta 8, Vbeta 12, and Vbeta 17 genes in CD4(+) T cells.[26] In 1995, Mathur and colleagues determined that P gingivalis and P intermedia increase the expression of Vbeta 5, Vbeta 6, Vbeta 8, and Vbeta 12.[27] As a result, specific T-cell genes are inherent in RA, and P gingivalis and P intermedia induce T-cell clones specific to them in an almost specific superantigen character. P gingivalis has a 60-kDa heat shock protein (hsp.GroEL). Due to the similarity of the P gingivalis hsp 60 molecule to the human hsp 60 molecule, it is a key molecule for GroEL homolog autoimmune reactions.[28] In another study conducted in Japan, approximately 70-kDa P melaninogenicaand P intermedia hsp proteins have been determined in periodontal disease processes.[29] Ueki and associates determined that P gingivalis GroEL and human hsp60 antibodies are found increasingly more often in periodontal patients compared to those without disease.[30] Hsp 70 antibodies have been detected in the synovial tissue of RA patients, and when the hsp 70 expression is induced with certain stress stimulating factors, proinflammatory cytokines (tumor necrosis factor-alpha, IL-1, IL-6) develop in the RA synovium.[31] Citrullination or deamination of arginine residues in autoantigenic proteins (profilaggrin/filaggrin, fibrinogen/fibrin, keratin, and vimentin) creates epitopes that are targeted by rheumatoid autoantibodies.[32] As can be clearly seen, arginine is the most important amino acid that allows proteins to gain an autoantigenic character, and P gingivalis has arginine-specific proteinases. The presence of autoantibodies against collagen II (CII) — the main component of hyaline cartilage — has been found previously in RA patients.[33,34] P gingivalis has collagenase activity, and it degrades all collagen molecules except for CII.[35] Within CII 263-270, lysines at 270 can be hydroxylated and further glycosylated with mono- or disaccharides, ie, with a beta-D-galactopyranosyl or an alpha-glucopyranosyl-(1-2)-beta-galactopyranosyl residue. Backlund and coworkers, using transgenic mice expressing human DR4 (DRB1*0401) and human CD, showed that the T cell produced by the postmutation glycosylation of CII influenced the tolerance level of the nominee cartilage-specific antigen and the predominant nature of these T cells specific to the CII epitope (263-270) in humanized transgenic mice and in RA patients.[36] Extracellular proteolysis and other posttranslational modifications of antigenic peptides may be critical in the establishment and perpetuation of autoimmune processes, and lysine hydroxylation is critical for T-cell activation.[37] P gingivalis, P intermedia, P melaninogenica , and B forsythus produce gingival tissue destruction and autoimmune responses in periodontitis patients. Heat shock proteins are remarkably immunogenic. T-cell and antibody responses in periodontitis confirm the infiltration of reactive T-cell clones into periodontitis lesions. These lesions demonstrate higher proliferative responses of peripheral blood mononuclear cells, cytokines (gamma-interferon, IL-4), and T-cell clonality. Immune responses to microbial heat shock proteins hsp60 are thought to initiate chronic inflammatory diseases; modulation of autoimmune responses may be a method to control pathogenesis. Higher levels of IgG and IgA antibodies against B forsythus and P intermedia were found in synovial fluid samples from patients with RA.[38] These findings indicate the presence of an active antibody response in synovial tissue and illustrate a potential connection between periodontal and inflammatory joint diseases. Gingival tissue infections should be considered in RA pathogenesis. Periodontal infections should be treated and prevented from becoming chronic. If successful results are observed against periodontal infections in clinical, radiologic, and laboratory data of the RA patients, the essential role of these bacteria in the etiology of RA can be proven.
Table. IgG Antibodies Against Oral Anaerobic Bacteria in the Sera of Patients With Rheumatoid Arthritis and Healthy Controls
References
|
*major impacts on health
*major impacts on quality of life
*much of the results are in your hands
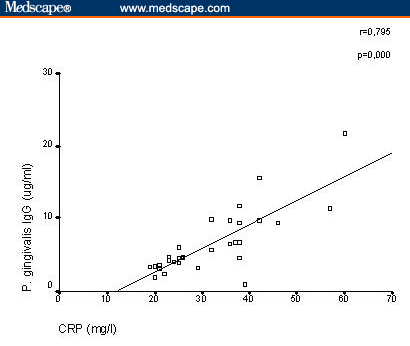 Figure 1.
Figure 1.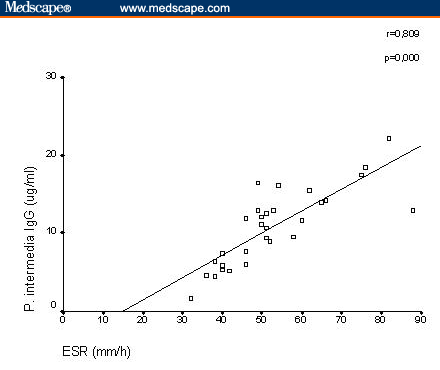 Figure 2.
Figure 2.