Physicians: periodontal attack and diabetes are intimately related. They make a vicious cycle. Hyperglycemia makes for lazy whitecells and poor healing. It exacerbates periodontal disease. And the reverse is also true: the worse periodontal disease gets, the harder it is to control blood glucose. The degree of gum attack correlates very tightly with levels of insulin resistance.If you suspect a patient has any diabetic tendencies, a dental referral is of premium importance.One good sign: Aetna Medical Insurance Company is covering 100% of periodontal care expenses for its insureds with diabetes. |
Gum Disease and Diabetes
 |
Diabetic patients are more likely to develop periodontal disease, which in turn can increase blood sugar and diabetic complications. |
People with diabetes are more likely to have periodontal disease than people without diabetes, probably because diabetics are more susceptible to contracting infections. In fact, periodontal disease is often considered the sixth complication of diabetes. Those people who don’t have their diabetes under control are especially at risk.
A study in the Journal of Periodontology found that poorly controlled type 2 diabetic patients are more likely to develop periodontal disease than well-controlled diabetics are.
Research has emerged that suggests that the relationship between periodontal disease and diabetes goes both ways – periodontal disease may make it more difficult for people who have diabetes to control their blood sugar.
Severe periodontal disease can increase blood sugar, contributing to increased periods of time when the body functions with a high blood sugar. This puts diabetics at increased risk for diabetic complications. Thus, diabetics who have periodontal disease should be treated to eliminate the periodontal infection.
This recommendation is supported by a study reported in the Journal of Periodontology in 1997 involving 113 Pima Indians with both diabetes and periodontal disease. The study found that when their periodontal infections were treated, the management of their diabetes markedly improved.
from http://www.perio.org:80/consumer/mbc.diabetes.htm
Family History of Diabetes + Perio Disease May Indicate Undiagnosed Diabetic Patient. Refer to MD
Diabetes in the dental office: using NHANES III* to estimate the probability of undiagnosed disease
Source: J Periodontal Res. 2007 Dec;42(6):559-65 Borrell LN, Kunzel C, Lamster I, Lalla E.
Department of Epidemiology, Columbia University College of Dental Medicine, Mailman School of Public Health, Columbia University, New York, NY Summary of research: • One third of diabetes cases remain undiagnosed.
• 60% of Americans see a dentist at least once per year.
• Dental professionals can screen patients for undiagnosed diabetes. Results and conclusions: • Periodontal patients with a family history of diabetes, hypertension and high cholesterol bear a probability of 27-53% of having undiagnosed diabetes.
• Dental office could provide an important opportunity to identify individuals unaware of their diabetic status. Key take-away • It is critical to have a thoroughly completed and reviewed health history, including family history of any type of diabetes.
• Dental providers should consider referring patients with a family history of diabetes and refractory periodontal disease to their physician for diabetic screening. Implementation strategies: • Incorporate a simple diabetes screening for patients with a family history of diabetes, hypertension, high cholesterol, and periodontal disease. **
• Identify and screen patients who are slow to heal after periodontal therapy or any dental surgical procedure.
• Involve the team in the development and use of the verbal skills to present this screening to those patients of concern. “Mrs. Smith, as you know Dr. Williams’ first concern is always for his patients and their overall health and wellness. With your family history of diabetes and your current health concerns coupled with your slow healing and recurrent periodontal infection, Doctor has asked me to assist you with a simple **diabetes screening questionnaire. It only takes a minute or two to complete. Doctor Williams will evaluate your responses and recommend a consultation with your physician if necessary.” *NHANES III, the Third National Health and Nutrition Examination Survey is a database collected from 34,000 persons by the CDC, 1988-1994. It was designed to obtain nationally representative information on the health and nutritional status of the U.S. population.
DEFEAT DIABETES® SCREENING TEST
Rate the incidence of each question with a
High / Yes = 10 points, Medium = 5 points, Low / No = 0 points
1. I go to the bathroom (urinate) often (every 2 to 3 hours): 10 5 0
2. I am always thirsty and/or hungry: 10 5 0
3. I am suddenly losing a lot of weight: 10 5 0
4. I am always fatigued (weak, tired) and/or drowsy: 10 5 0
5. I am irritable and have mood changes: 10 5 0
6. I am nauseous and/or vomit often: 10 5 0
7. I have blurred vision: 10 5 0
8. I have a tingling or numbness in my legs, feet or fingers: 10 5 0
9. I have frequent or recurring skin, gum and/or urinary tract infections: 10 5 0
10. I have frequent itching of my skin and/or genitals: 10 5 0
11. I have slow healing of cuts and bruises: 10 5 0
12. My family history shows diabetes: Yes no / don’t’ know
13. My Age is: >65 45-65 <45
14. I am: Obese (>20% overweight) Overweight Not Overweight
15. For women, do you have a history of gestational diabetes (the type that occurs
during pregnancy) or have delivered a baby over 9 pounds. Yes No
16. If you are Asian, Black, Hispanic/Latino, Pacific Islander or Native American,
medical data shows that within these ethnic groups there are extremely high
diabetic populations.
I am Asian, Black, Hispanic/Latino, I am Caucasian
Pacific Islander or Native American
17. Indications of Acanthosis Nigricans (AN), a skin condition characterized by
darkened, velvety and/or thickened skin patches. Yes No
18. Necrobiosis Lipoidica Diabeticorum (NLD), slightly raised shiny red-brown
patches on my lower legs, mostly in women. Yes No
Scoring Your DEFEAT DIABETES® SCREENING TEST
35 or More Points: You have scored very high and should seek medical evaluation!
20 to 30 Points: You probably have pre-diabetes and should get tested.
0 to 15 Points: You are at low risk for having diabetes.
from: http://www.perioeducation.com/members/courses/1543/PDF/PerioFogz_diabetes.pdf
Treating gum disease linked to lower medical costs for patients with diabetes
University of Michigan, December 23, 2008
ANN ARBOR, Mich.—A new report suggests that treating gum disease in patients who have diabetes with procedures such as cleanings and periodontal scaling is linked to 10 to 12 percent lower medical costs per month.
The findings are encouraging but the study was not designed to firmly establish cause and effect, said George Taylor, University of Michigan associate professor of dentistry, who also has an appointment in epidemiology in the U-M School of Public Health. Taylor led the research project to investigate whether routine, non-surgical treatment for gum disease is linked to lower medical care costs for people with diabetes.
In periodontal disease, the body reacts to the bacteria causing the gum infection by producing proteins or chemicals called inflammatory mediators. Ulcers and open sores in the gums become passageways for these proteins and for the bacteria themselves to enter the body’s blood circulation. These inflammatory mediators, as well as some parts of the bacteria, prevent the body from effectively removing glucose, or sugar, from the blood. The higher level of blood sugar is known as poor diabetes control. Poor diabetes control leads to serious diabetes complications such as vision disorders, cardiovascular and kidney disease and amputations, among others. “Cleanings and other non-surgical periodontal treatment remove the harmful bacteria,” Taylor said. “We believe this helps prevent the body from producing those harmful chemicals that can enter the systemic circulation and contribute to poorer diabetes control.”
Blue Care Network provided U-M researchers data from 2,674 patients aged 18-64 who were enrolled in BCN between 2001 and 2005 and had at least 12 consecutive months of medical, dental, and pharmaceutical coverage.
“We found insured adults with diabetes in Michigan who received routine periodontal treatment, such as dental cleanings and scaling, have significantly lower medical care costs than those who do not,” Taylor said. “These results could be meaningful to individuals, employers, health care providers and insurers.” The study showed that medical care costs decreased by an average of 11 percent per month for patients who received one or two periodontal treatment procedures annually compared to those who received none. For patients receiving three or four annual treatments, costs decreased nearly 12 percent. The study also showed that combined medical and pharmaceutical monthly costs were 10 percent lower for patients who received one or two periodontal procedures annually.
“The results of our analyses provide additional evidence supporting a beneficial role for periodontal treatment in improving overall health for people with diabetes,” Taylor said. The findings could fuel changes in policies and practices for diabetes patients and their insurers.
The research was supported by a grant from the Blue Cross Blue Shield of Michigan Foundation. Taylor’s team includes: Wenche Borgnakke, senior research associate in health sciences; Michael Manz, senior research associate in health sciences; and Tammie Nahra, assistant research scientist.
AAP-Commissioned Review
Diabetes Mellitus and Periodontal Diseases
Brian L. Mealey* and Thomas W. Oates*
Background: The purpose of this review is to provide the reader with practical knowledge concerning the relationship between diabetes mellitus and periodontal diseases. Over 200 articles have been published in the English literature over the past 50 years examining the relationship between these two chronic diseases. Data interpretation is often confounded by varying definitions of diabetes and periodontitis and different
clinical criteria applied to prevalence, extent, and severity of periodontal diseases, levels of glycemic control, and complications associated with diabetes.
Methods: This article provides a broad overview of the predominant findings from research published in English over the past 20 years, with reference to certain ‘‘classic’’ articles published prior to that time.
Results: This article describes current diagnostic and classification criteria for diabetes and answers the following questions: 1) Does diabetes affect the risk of periodontitis, and does the level of metabolic control of diabetes have an impact on this relationship? 2)Do periodontal diseases affect the pathophysiology of diabetes mellitus or the metabolic control of diabetes? 3) What are the mechanisms by which these two
diseases interrelate? and 4) How do people with diabetes and periodontal disease respond to periodontal treatment? Conclusions: Diabetes increases the risk of periodontal diseases, and biologically plausible mechanisms have been demonstrated
in abundance. Less clear is the impact of periodontal diseases on glycemic control of diabetes and the mechanisms through which this occurs. Inflammatory periodontal diseases may increase insulin resistance in a way similar to obesity, thereby aggravating glycemic control. Further research is needed to clarify this aspect of the relationship between periodontal diseases and diabetes. J Periodontol 2006;77:1289-1303.
…
METHODS
The information presented in this review is based on a survey of English language literature primarily over the last 20 years, although certain ‘‘classic’’ articles are referenced from before the 1980s. The literature search was conducted using the National Library of Medicine’s Entrez PubMed search engine. The article does not contain an exhaustive article by-article review of the literature but, instead, provides a broad overview of the predominant findings from research. The article does not seek to analyze statistically any of the data from the reviewed articles, but relies on the original data analysis and author interpretation. Several references are cited from the medical
literature and are not meant to be inclusive of all or even a substantial part of the medical literature available on the subject of diabetes mellitus. Diabetes mellitus is a clinically and genetically
* Department of Periodontics, University of Texas Health Science Center at San Antonio, San Antonio, TX.
J Periodontol • August 2006- 1289
heterogeneous group of disorders affecting the metabolism of carbohydrates, lipids, and proteins.1 The characteristic feature of diabetes is an abnormal elevation in blood glucose levels. Hyperglycemia is due to a deficiency of insulin secretion caused by pancreatic b-cell dysfunction and/or insulin resistance in liver and muscle.2 This metabolic dysregulation is often associated with alterations in adipocyte metabolism. Diabetes is a syndromein which chronic hyperglycemia leads to long-term damage to various organs including the heart, eyes, kidneys, nerves, and vascular system.
DIABETES EPIDEMIOLOGY AND CLASSIFICATION
Diabetes affects ;21 million Americans, including over 9% of the adult population.3,4 Approximately 6 million of these individuals have the disease but are undiagnosed.5 The prevalence of diabetes is increasing annually in the United States and varies by age and
racial category, with older individuals, Native Americans, Hispanics, and non-Hispanic blacks more commonly having diabetes than younger individuals and non-Hispanic whites. The incidence of diabetes is also increasing annually. In 2002, ;1.3 million new cases of diabetes were diagnosed, an increase of 500,000 new cases per year since 1998, when the incidence was 800,000 cases.5 The rise in prevalence and incidence
of diabetes is directly related to increasing obesity rates in the American population.5 About 85% to 90% of diabetic cases are type 2 diabetes, whereas type 1 diabetes constitutes5%to 10%of patients. Gestational diabetes and secondary forms of diabetes
associated with other conditions such as pancreatic disease, drug therapies, and endocrine disorders account for the remainder of cases. The current classification of diabetes is based upon the pathophysiology of each form of the disease.2 Type 1 diabetes results from cellular mediated autoimmune destruction of pancreatic b-cells, usually leading to total loss of insulin secretion. Markers of autoimmune destruction have been identified and can be used for diagnosis or risk assessment.2 Type 1 diabetes is usually present in children and adolescents, although some studies demonstrated 15% to 30% of all cases being diagnosed after 30 years of age.6 In older type 1 patients, the b-cell destruction occurs more slowly than in children, with a less abrupt onset of symptoms. This demonstrates that the pace and extent of cellular destruction can occur at a different rate from patient to patient. The lack of insulin production in patients with type 1 diabetes makes the use of exogenous insulin necessary to sustain life, hence the former name ‘‘insulin-dependent diabetes.’’2 In the absence of insulin, these patients develop ketoacidosis, a life-threatening condition. Type 2 diabetes, previously called non–insulindependent diabetes, results from insulin resistance, which alters the use of endogenously produced insulin at the target cells.1,2 Type 2 patients have altered insulin
production as well; however, autoimmune destruction of b-cells does not occur as it does in type 1, and patients retain the capacity for some insulin production. Because the type 2 patient still produces insulin, the incidence of ketoacidosis is very low compared to type 1; however, ketoacidosis can occur in association with the stress of another illness such as infection. Type 2 patients can be undiagnosed for many years because the hyperglycemia appears gradually and often without symptoms.7 In many patients, especially early in the disease process, pancreatic insulin production is actually increased to compensate for insulin resistance. As the condition progresses, pancreatic insulin production may diminish over time due to the prolonged increase in secretory
demand caused by the insulin resistance.8 Insulin secretion becomes insufficient to compensate for insulin resistance. Although type 2 patients do not need insulin treatment to survive, insulin is often taken as part of the medical management of type 2 diabetes.
Most patients with type 2 diabetes are obese or have an increased percentage of body fat distributed predominantly in the abdominal region.1 The normal body mass index (BMI) is under 25 kg/m2, whereas a BMI between 25 and 30 kg/m2 is defined as overweight, and a BMI of over 30 kg/m2 is defined as obese. Adipose tissue plays an important role in the development of insulin resistance.9 Elevated circulatinglevels of free fatty acids (FFA) derived from adipocytes contribute to insulin resistance by inhibiting
glucose uptake, glycogen synthesis, and glycolysis and by increasing hepatic glucose production.10 Insulin resistance often improves with weight reductionand pharmacological treatment but is generally not restored to normal.
Gestational diabetes complicates ;4% of all pregnancies in the Unites States, but the prevalence can range from 1% to 14% of pregnancies, depending on the population studied.11 Gestational diabetes usually has its onset in the third trimester of pregnancy, and adequate treatment will reduce perinatal morbidity. Most women with gestational diabetes return to a normoglycemic state after parturition; however, a history of gestational diabetes significantly increases the risk of subsequently developing type 2 diabetes. Under normal conditions, insulin secretion is increased by 1.5- to 2.5-fold during pregnancy, reflecting a state of insulin resistance.12 A woman with a limited b-cell
reserve may be incapable of increasing insulin production to compensate for her insulin-resistant state,resulting in hyperglycemia.
Diabetes Mellitus and Periodontal Diseases Volume 77 • Number 8 1290
Some individuals have glucose levels that do not meet the criteria for diabetes but are too high to be considered normal. Members of this group have a condition called ‘‘prediabetes,’’ a term which encompasses both impaired fasting glucose (IFG) and impaired glucose tolerance (IGT).2 These patients are usually normoglycemic but demonstrate elevated blood glucose levels under certain conditions. People whose hyperglycemia is limited to periods of fasting have impaired fasting glucose, whereas those whose hyperglycemia occurs after a glucose load have impaired glucose tolerance. Both impaired fasting glucose and impaired glucose tolerance are strong
predictors for future development of type 2 diabetes; furthermore, impaired glucose tolerance is a significant predictor of myocardial infarction and stroke.
13
DIABETES: DIAGNOSTIC CRITERIA AND EVALUATION OF GLYCEMIC CONTROL
In 1998, the World Health Organization adopted the diagnostic parameters for diabetes established by the American Diabetes Association.14 Currently, there are three ways to diagnose diabetes.2 Because a single abnormal laboratory test is not sufficient to establish a diagnosis, any positive laboratory value must be confirmed on a different day: 1) symptoms of diabetes plus casual plasma glucose concentration ‡200 mg/dl (‡11.1 mmol/l). Casual is defined as any time of day without regard to the time since the last meal. The classic symptoms of diabetes include polyuria, polydipsia, and unexplained weight loss; 2) fasting plasma glucose ‡126 mg/dl (‡7.0 mmol/l).
Fasting is defined as no caloric intake for at least 8 hours; and 3) 2-hour postload glucose ‡200 mg/dl (‡11.1 mmol/l) during an oral glucose tolerance test. The test should be performed using a glucose load containing the equivalent of 75 g anhydrous glucose
dissolved in water. The normal fasting plasma glucose level is <100mg/dl (5.6 mmol/l). Impaired fasting glucose is diagnosed when the fasting plasma glucose level is ‡100
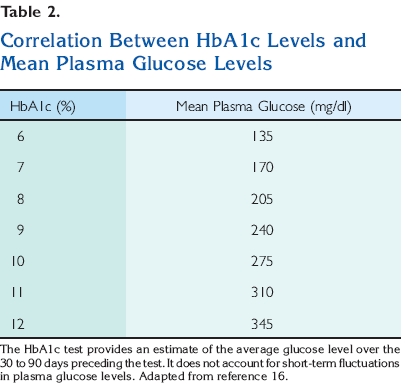
mg/dl but £125 mg/dl (between 5.6 and 6.9 mmol/l). Impaired glucose tolerance can only be diagnosed after an oral glucose tolerance test. A normal 2-hour postload glucose level is <140 mg/dl (7.8 mmol/l). Impaired glucose tolerance is diagnosed when the 2-hour postload plasma glucose concentration is ‡140 mg/dl but £199 mg/dl (between 7.8 and 11.1 mmol/l) (Table 1). In a patient with diagnosed diabetes, the hemoglobin A1c test (HbA1c) is used to monitor the patient’s overall glycemic control. It is not recommended for diagnosis because there is not a gold standard assay for the HbA1c and because many countries do not have ready access to the test. Glycohemoglobin is formed continuously in erythrocytes as the product of a nonenzymatic reaction between glucose and the hemoglobin protein, which carries oxygen. The binding of glucose to hemoglobin is highly stable; therefore, hemoglobin remains glycated for the life span of the erythrocyte, ;123 – 23 days.15 The HbA1c test is used to measure glycohemoglobin levels and provides an estimate of the average blood glucose level over the preceding 30- to 90-day period. Higher average blood glucose levels are reflected in higher HbA1c
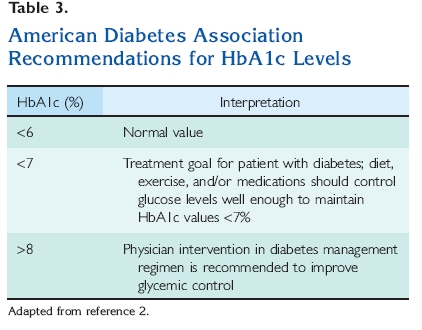
values16 (Table 2). The normal HbA1c is <6%2 (Table 3). HbA1c levels correlate well with the development of diabetic complications and may become established as a test for the diagnosis of diabetes at some time in the future.17

In the presence of hyperglycemia, other serum proteins beside hemoglobin are also glycated. Measurement of these glycated proteins can be used as an alternative to the HbA1c for assessment of glycemic control over time.2 For example, albumin is a serum
protein with a half-life of 2 to 3 weeks. The fructosamine test measures glycated albumin, and this test reflects glycemic control over a shorter interval (weeks) than the HbA1c test (months). The fructosamine test is sometimes used when an objective measurement that reflects a shorter period of time is needed, for example, during pregnancy, initiation of
a new therapy, or a medical illness. Itmayalso be used in instances when the HbA1c test may not be reliable, such as when anemia is present. The normal range for the fructosamine test is between 200 and 300 mmol/l.
EFFECTS OF DIABETES ON THE PERIODONTIUM
Examination of the available data reveals strong evidence that diabetes is a risk factor for gingivitis and periodontitis, and the level of glycemic control appears to be an important determinant in this relationship. 18,19 One must use caution in evaluating the
research because studies examined diverse populations, often lacked controls or had small numbers of subjects, defined diabetes and glycemic control in various ways, and used different periodontal parameters to describe the clinical conditions present. Although some authors have not found a significant association between diabetes and gingival inflammation, 20 in many studies, the prevalence and severity of
gingivitis has been demonstrated to be higher in individuals with diabetes. In children with type 1 diabetes, the prevalence of gingivitis was greater than in nondiabetic children with similar plaque levels.21 Twice as many sites had gingival inflammation in children with diabetes compared to non-diabetic control children with similar plaque levels.22 Poor metabolic control can increase the severity of gingival inflammation in diabetic children,23 whereas improvement in glycemic control may be associated with decreased gingival inflammation.24,25 In adults with type 1 diabetes, the overall degree of gingival inflammation was similar between diabetic subjects as a whole and non-diabetic control subjects with similar plaque accumulation.26 However, when diabetic patients in this study were stratified according to their level of glycemic control, significantly greater gingival bleeding was seen in poorly controlled diabetic patients than in either well-controlled diabetic subjects or nondiabetic controls. The number of bleeding sites decreased as glycemic control improved.26 Greater gingival inflammation was also seen in adults with type 2 diabetes than in non-diabetic controls, with the highest level of inflammation in subjects with poor glycemic control.27 A longitudinal experimental gingivitis study28 showed more rapid and pronounced development of gingival inflammation in relatively well-controlled adult type 1 diabetic subjects than in
non-diabetic controls, despite similar levels of plaque accumulation and similar bacterial composition of plaque, suggesting a hyperinflammatory gingival response in diabetes. These studies suggest that the presence of diabetes is often, but not always, associated
with increased gingival inflammation. In addition, the level of glycemic control may play a role in the gingival response to bacterial plaque in people with diabetes.
The preponderance of evidence suggests that diabetes also increases the risk of periodontitis. A thorough meta-analysis concluded that the majority of studies demonstrate a more severe periodontal conditionin diabetic adults than in adults without diabetes.18 These studies included over 3,500 diabetic adults and clearly demonstrated a significant association between periodontitis and diabetes. Diabetes has been associated with an increased risk of periodontitis even at a young age. In a group of 263 type 1 diabetic patients compared to 59 nondiabetic siblings and 149 non-diabetic unrelated controls, periodontitis was not seen among any of the subjects under the age of 12.21 However, between 13 and 18 years of age, 13.6% of the diabetic individuals had periodontitis, and the prevalence increased to 39% among those aged 19 to 32 years. By comparison, the prevalence in non-diabetic control subjects was <3%.
Epidemiologic studies in diabetic adults have often shown an increase in extent and severity of periodontitis. 29-32 In the Pima Indians of Arizona, a population with the highest occurrence of type 2 diabetes in the world, the prevalence and severity of attachment loss and bone loss was greater among diabetic subjects
than among non-diabetic control subjects in all age groups.30,31 In a multivariate risk analysis, diabetic subjects had 2.8- to 3.4-fold increased odds of having periodontitis compared to non-diabetic subjects after adjusting for the effects of confounding variables such as age, gender, and oral hygiene measures. Smaller cross-sectional and case-control studies generally confirmed a greater risk of attachment loss and bone loss in diabetic adults.27,33-37 Longitudinal research has also shown an increased risk of progressive periodontal destruction in people with diabetes. In a study of the Pima Indians, the incidence and prevalence of periodontal disease were determined in 2,273 subjects 15 years of age or older.38 The prevalence of periodontitis was 60% in subjects
with diabetes and 36% in those without diabetes. The incidence was determined in a subset of 701 subjects 15 to 54 years old, with little or no evidence of periodontitis at baseline. Following these subjects for an average of over 2.5 years, the incidence of periodontitis was 2.6-fold higher in diabetic subjects than in non-diabetic patients.38 In another 2-year longitudinal study, subjects with type 2 diabetes had a fourfold increased risk of progressive alveolar bone loss compared to non-diabetic subjects.39 The relationship between metabolic control of diabetes and periodontal disease is difficult to define conclusively. 19 Research suggests that this association is similar to the association between glycemic control and the classic complications of diabetes such as retinopathy and nephropathy; namely, there is significant heterogeneity in the diabetic population. Thus, although poor control of diabetes clearly increases the risk of diabetic complications, there are many poorly controlled diabetic individuals without major complications. 40,41 Conversely, good control of diabetes greatly decreases the risk of diabetic complications, but there are people with well-controlled diabetes who suffer major diabetic complications nonetheless. In a similar fashion, the body of evidence suggests that some diabetic patients with poor glycemic control develop extensive periodontal destruction, whereas others do not. On the other hand, many well-controlled diabetic patients have excellent periodontal health, but others develop periodontitis.
In a large epidemiologic study in the United States, adults with poorly controlled diabetes had a 2.9-fold increased risk of having periodontitis compared to non-diabetic adult subjects; conversely,well-controlled diabetic subjects had no significant increase in the risk of periodontitis.42 In a cross-sectional study of patients who had type 1 diabetes for a mean duration of over 16 years, subjects with poor glycemic control had more interproximal attachment loss and bone loss than well-controlled subjects.43 Similar results have been found in other studies in which the percentage of deep periodontal pockets and the prevalence of severe attachment loss increased as the glycemic control worsened.44,45 Type 1 diabetic subjects with poor metabolic control over the preceding 2 to 5 years had a significantly greater prevalence of deep probing depths and advanced attachment loss than subjects with good glycemic control.32 Likewise, poorly controlled
diabetic subjects had significantly greater bone loss and attachment loss than well-controlled diabetic subjects over a 2- to 3-year follow-up period.46,47 In longitudinal Pima Indian studies, poor glycemic control of type 2 diabetes was associated with an 11-fold increased risk of progressive bone loss compared to non-diabetic controls, whereas well-controlled diabetic subjects had no significant increase in risk.39 Thus, metabolic control of diabetes may be an important variable in the onset and progression of periodontal disease.Other studies have given only marginal support to the relationship between glycemic control and the extent or severity of periodontitis, whereas some have
shown no relationship. In a study of 118 diabetic subjects and 115 healthy controls, deeper probing depths and greater gingival inflammation, bleeding on probing, and attachment loss were seen in those with diabetes; however, the level of glycemic control among the diabetic subjects did not correlate to the periodontal parameters measured.33 Another study found a trend toward an increasing prevalence of alveolar bone loss as glycemic control worsened.36 The mean percentage of sites with >15% bone loss went from 28% in well-controlled type 1 diabetic subjects to 44% in poorly controlled subjects. However, the difference did not reach statistical significance, perhaps due to the small size of the study population. Some studies found no evidence of a relationship between
glycemic control and periodontal status.
48,49 MECHANISMS BY WHICH DIABETES MAY INFLUENCE THE PERIODONTIUM
To validate a relationship between diabetes and periodontal diseases, biologically plausible mechanisms must be evident to explain the pathobiology of the interactions. A large evidence base is available to describe these potential mechanisms, many of which
are strikingly similar to those associated with the classic diabetic complications, including retinopathy, nephropathy, neuropathy, macrovascular diseases, and altered wound healing. The strength of the evidence has led some to suggest that periodontitis should be listed among the ‘‘classic’’ complications of diabetes.50 Although bacteria are necessary for periodontal diseases to occur, there are few differences in the subgingival
microflora between diabetic and non-diabetic patients with periodontitis, although some early J Periodontol • August 2006 Mealey, Oates 1293 studies reported higher proportions of Capnocytophaga species in those with diabetes.51 Most culture studies show that the bacterial microflora at periodontally diseased sites in diabetic subjects is similar to the microflora at similarly diseased sites in non-diabetic subjects.49,52 Likewise, no significant differences in the subgingival microflora were seen between type 1 diabetic children and their non-diabetic siblings.20 These studies involved the use of culture techniques to identify bacteria; it is unknown whether newer identification techniques will confirm the similarity in the subgingival bacterial microflora between people with and without diabetes. However, the apparent lack of significant differences in potential pathogens suggests that alterations in the host immunoinflammatory response may have a major influence on the increased prevalence and severity of periodontal destruction seen in diabetes. The function of immune cells, ncluding neutrophils, monocytes, and macrophages, is altered in diabetes.51 Neutrophil adherence, chemotaxis, and phagocytosis are often impaired, which may inhibit bacterial killing in the periodontal pocket and significantly increase periodontal destruction.
53,54 Although the function of neutrophils is often diminished in diabetes, the monocyte/macrophage cell line may exhibit upregulation in response to bacterial antigens. The hyperresponsiveness ofmonocytes/macrophages results in significantly increased production of proinflammatory cytokines and mediators.55-57 Peripheral blood monocytes from diabetic subjects produce elevated levels of tumor necrosis factor-alpha (TNF-a) in response to antigens from Porphyromonas gingivalis compared to monocytes from non-diabetic control subjects.55 These findings are supported in a diabetic animal model in which P. gingivalis inoculation produced a prolonged inflammatory response.
56 Interestingly, this prolonged inflammatory response was found to be independent of the pathogenic components of the inoculated organisms and directly related to TNF stimulation. Because gingival crevicular fluid is a serum transudate, elevated serum levels of inflammatory mediators associated with diabetes are reflected in similarly increased levels of these mediators in gingival crevicular fluid.57 The level of inflammatory cytokines in the gingival crevicular fluid is also related to glycemic control of diabetes. In a study of diabetic subjects with periodontitis, those with HbA1c levels over 8% had crevicular fluid levels of interleukin-1 beta (IL-1b) almost twice as high as subjects with HbA1c levels <8%.58 The net effect of these host defense alterations in diabetes is an increase in periodontal inflammation, attachment loss, and bone loss.
The increased levels of periodontal attachment and bone loss seen in diabetic patients may be associated with the alterations in connective tissue metabolism that uncouple the resorptive and formative responses. Impaired osseous healing and bone turnover in association with hyperglycemia have been demonstrated in a number of studies.59-63 The effects of a hyperglycemic state include inhibition of osteoblastic cell proliferation and collagen production that result in reduced bone formation and diminished mechanical properties of the newly formed bone.64-67 Interestingly, using a murine model, the reduced expression of two genetic markers of osteoblastic differentiation,
Cbfa1 and Dlx5, found in response to hyperglycemia were reversed with insulin treatment controlling the hyperglycemia.66 There is additional evidence emerging that decreases in matrix-producing cells critical to maintaining the periodontium, including fibroblasts and osteoblasts, occur due to an increased rate of apoptosis in a hyperglycemic state in response to P. gingivalis infection.68-70 Together, the diminished levels of proliferation and differentiation and increased levels of cell death provide a compelling argument for the greater propensity of diabetic patients to have more severe periodontal attachment loss due to inadequacies in the formative aspects of connective tissue metabolism relative to the degradation and remodeling of tissues of the attachment apparatus. Increased plasma glucose levels are also reflected in elevated gingival crevicular fluid glucose levels in diabetic individuals.71 Because the periodontal pocket is a site of persistent bacterial wounding, an intact wound-healing response is critical to maintain tissue health. High glucose levels in the gingival crevicular fluid may directly hinder the wound-healing capacity of fibroblasts in the periodontium by inhibiting attachment and spreading of these cells that are critical to wound healing and normal tissue turnover.72 Microvascular changes are a hallmark of many diabetic complications.73 The structural changes that characterize diabetic angiopathy include abnormal growth and impaired regeneration of vessels. The changes seen in the microvasculature of the retina, glomerulus, and other end organs in people with diabetic complications also occur in the periodontium. 74,75 In individuals with sustained hyperglycemia, proteins become irreversibly glycated to form advanced glycation end products (AGEs).76 These stable carbohydrate-containing proteins have multiple effects on cell-to-cell and cell-to-matrix interactions and are commonly thought to be a major link
between the various diabetic complications. The formation of AGEs also occurs in the periodontium, and higher levels of periodontal AGE accumulation are found in those with diabetes than in non-diabetic subjects. 77 AGEs often form on collagen, increasing collagen cross-linking and resulting in the formation Diabetes Mellitus and Periodontal Diseases Volume 77 • Number 8 1294 of highly stable collagen macromolecules. These
molecules accumulate in tissues due to their resistance to normal enzymatic degradation and tissue turnover.76 AGE-modified collagen accumulates in the walls of larger blood vessels, thickening the vessel wall and narrowing the lumen. In addition, AGE-modified
vascular collagen has an affinity for low-density lipoprotein (LDL) and causes the accumulation of LDL in the vessel wall, contributing to atherosclerotic changes characteristic of macrovascular complications of diabetes.78 The basement membranes of endothelial cells also accumulate AGE-modified collagen macromolecules, which can result in increased basement membrane thickness in the microvasculature, altering normal homeostatic transport across the membrane.78 This increased basement membrane thickness is seen in the blood vessels of the periodontiumin people with diabetes.74AGEformation is also associated with increased production of vascular
endothelial growth factor (VEGF), a multifunctional cytokine that induces neovascularization and plays amajor role in microvascular complications of diabetes.
79,80 Elevated VEGFhas been detected in serum of diabetic individuals and in all major tissues affected by diabetic vasculopathies. A recent study found elevated VEGF expression in the gingival tissues of diabetic subjects compared to non-diabetic controls,
again demonstrating the similarities between the periodontium and other end organs affected by diabetes.81 AGE-collagen levels have been well correlated with duration of diabetes, diabetic complications, and glycemic control. 82 Furthermore,improved glycemic control has been associated with reduced AGE-collagen formation.83,84 Mechanistically, AGE–bone collagen mayinfluence cellular, structural, and functional characteristics leading to alterations in bone metabolism. 85-87 Altered levels of glycation in bone collagen appear to affect bone turnover, such that bone formation is reduced with elevated levels ofAGEcollagen.88 This effect has been associated with altered osteoblastic differentiation and extracellular matrix production.89,90 The effects of AGE-collagen are not as clear regarding bone resorption. Although several studies
documented increased levels of osteoclast numbers, resorptive markers, and bone resorption,91-93 there are a number of studies that suggest decreased bone resorption may occur.94-96 As such, the role of AGEs on the resorptive aspects of bone metabolism are likely most relevant to the inflammatory response. AGEs activate a receptor known as ‘‘receptor for AGEs’’ (RAGE) found on the surface of smooth muscle
cells, endothelial cells, neurons, and monocytes/ macrophages.97 This receptor is found in the periodontium, and a 50% increase in mRNA for RAGE was identified in the gingival tissues of type 2 diabetic subjects compared to non-diabetic controls.77,98 Hyperglycemia results in increased RAGE expression and AGE-RAGE interaction on the endothelium, causing an increase in vascular permeability and thrombus formation.73,97 The AGE-RAGE interaction on monocytes increases cellular oxidant stress and activates the transcription factor nuclear factorkappa B (NF-kB), which alters the phenotype of the monocyte/macrophage and results in the increased production of proinflammatory cytokines such as IL-1b and TNF-a.78,97 This increased production of proinflammatory cytokines is critical to the chronic inflammatory process in the formation of atheromatous lesions in the larger blood vessels.99 Increased oxidant stress has also been demonstrated in the gingiva of diabetic subjects in association with an increased accumulation of AGEs.77 It is this interaction between the receptor RAGE and AGEs in periodontal tissues that is thought to explain, in part, the marked elevation in gingival crevicular fluid levels of IL-1b, TNF-a,
and prostaglandin E2 (PGE2) seen in diabetic subjects compared to non-diabetic individuals.58 These proinflammatory cytokines contribute to the pathogenesis of periodontal diseases and probably play a major role in patients with diabetes, especially
when glycemic control is poor. In diabetic animal models, blocking the receptor RAGE decreases TNF-a, IL-6, and matrix metalloproteinase (MMP) levels in the gingiva, diminishes AGE accumulation in periodontal tissues, and decreases alveolar bone
loss in response to P. gingivalis.100 Changes in collagen synthesis,maturation, and homeostatic turnover are common in diabetes. These changes can contribute to the pathogenesis of periodontal diseases and to alterations in wound healing because collagen is the major structural protein in the periodontium. Human gingival fibroblasts produce decreased amounts of collagen and glycosaminoglycans in high-glucose environments.101 Diabetic animals exhibit a decreased rate of collagen production
that can be restored by the administration of insulin to normalize plasma glucose levels.102 In addition to decreased synthesis, newly formed collagen is susceptible
to degradation byMMPs such as collagenase, which are elevated in diabetic tissues, including the periodontium.103,104 In diabetes, a greater proportion of tissue collagenase is in an active form compared to non-diabetic individuals, in whom a greater proportion is in latent form.104 In contrast to the effects that elevated MMPs have on newly synthesized collagen, existing collagen becomes highly cross-linked in the presence of AGEs, decreasing its solubility.76 The result of these changes in collagen metabolism is an alteration in normal homeostatic collagen turnover in which recently synthesized collagen is rapidly J Periodontol • August 2006 Mealey, Oates 1295
degraded by elevated levels of active MMPs, whereas highly cross-linked AGE-modified collagen macromolecules accumulate in the tissues. This change in homeostasis may alter wound healing responses to chronic microbial wounding of the periodontium.
EFFECTS OF PERIODONTAL DISEASES ON THE DIABETIC STATE
Periodontal diseases can have a significant impact on the metabolic state in diabetes. The presence of periodontitis increases the risk of worsening of glycemic control over time. For example, in a 2-year longitudinal trial, diabetic subjects with severe periodontitis at baseline had a six-fold increased risk of worsening of glycemic control over time compared to diabetic subjects without periodontitis.105 Periodontitis may also
be associated with an increased risk of other diabetic complications, as seen in a longitudinal case-control study in which 82% of diabetic patients with severe periodontitis experienced the onset of one or more major cardiovascular, cerebrovascular, or peripheral vascular events compared to only 21% of diabetic subjects without periodontitis.106 Because cardiovascular diseases are so widely prevalent in people with
diabetes, a recent longitudinal trial examined the effect of periodontal disease on overall mortality and cardiovascular disease–relatedmortality in more than 600 subjects with type 2 diabetes.107 In subjects with severe periodontitis, the death rate from ischemic
heart disease was 2.3 times higher than in subjects with no periodontitis or mild periodontitis, and the mortality rate from diabetic nephropathy was 8.5 times higher in the severe periodontitis group after accounting for other known risk factors. The overall
mortality rate from cardio-renal disease was 3.5 times higher in subjects with severe periodontitis. Intervention trials have been performed to assess the potential effects of periodontal therapy on glycemic control in people with diabetes. The first such study, a case series published in 1960, showed that type 1 diabetic patients with periodontitis had a reduction in required insulin doses following scaling and root planing, localized gingivectomy, and selected tooth extraction combined with systemic procaine penicillin G and streptomycin.108 In more recent times, treatment has usually consisted of scaling and root planing either alone or in combination with adjunctive systemic tetracycline antibiotics. Tetracyclines decrease the production of MMPs such as collagenase and are a logical choice for study because collagenase production is often elevated in diabetic patients.109 Several studies of type 1 and type 2 diabetic subjects
with severe periodontitis have shown improvements in glycemic control following scaling and root planing combined with systemic doxycycline therapy. 110-112 In these studies, periodontal treatment was associated with a reduction in HbA1c levels of ;10% between pretreatment baseline values and 2- to 3-month post-treatment values. Another study in
older, poorly controlled type 2 diabetic subjects who received scaling and root planing plus adjunctive doxycycline showed a significant improvement in periodontal health but only a non-significant reduction in HbA1c values.113 Some studies in which patients
received scaling and root planing without adjunctive systemic antibiotics likewise showed improved periodontal health but no significant change in glycemic control.114,115 Conversely, other studies showed significant improvement in glycemic control when periodontal therapy consisted of scaling and root planing alone.116,117 One study even showed better improvement in glycemic control in a diabetic group treated with scaling and root planing alone compared to diabetic subjects treated with scaling and root planing plus systemic amoxicillin/clavulanic acid.118 The effect of periodontal therapy on glycemic control is often mirrored by changes in clinical parameters of periodontal inflammation. For example, in a study of well-controlled type 2 diabetic patients with gingivitis or mild periodontitis, periodontal treatment was limited to scaling and root planing without systemic antibiotics.117 A control group of diabetic subjects with a similar periodontal status received no treatment. Three months after therapy, the treated subjects had a 50% reduction in the prevalence of gingival bleeding, from 55% of sites at baseline to 24% of sites post-treatment. These same subjects had a significant
reduction in mean HbA1c from 7.3% to 6.5%. As expected, the untreated control group had no change in gingival bleeding 3 months after baseline (51% of sites at baseline; 52% post-treatment), nor did they have any improvement in HbA1c (baseline, 7.0%; follow-up, 7.3%). Thus, significant changes in glycemic control may accompany clinically evident improvement in gingival inflammation following periodontal therapy. These conflicting data are difficult to interpret, especially given the wide range of medical treatment regimens used by study populations, which may confound changes related to resolution of periodontal inflammation. 119 In most studies, there is significant variation in glycemic control changes of individual subjects after periodontal therapy. For example, responses can range from major reductions in HbA1c values of 1 to 2 absolute percentage points or more, whereas in other subjects receiving the same therapy, HbA1c values may change little or may even worsen.116 A recent meta-analysis of 10 intervention trials included 456 patients.119 After periodontal therapy, the weighted average decrease in absolute HbA1c values was ;0.4%, but this was not found to Diabetes Mellitus and Periodontal Diseases Volume 77 • Number 8 1296 be statistically significant. The addition of adjunctive systemic antibiotics to the mechanical therapy regimen resulted in an average absolute reduction of 0.7%. Again, this reduction did not achieve a level of statistical significance. The authors of this metaanalysis pointed out numerous problems with existing
studies including inadequate sample sizes, mixing of subjects with type 1 and type 2 diabetes, and confounding effects of smoking, body mass index, and medications, among others. Further studies are required to determine whether periodontal therapy provides a significant benefit on glycemic control.
MECHANISMS BY WHICH ERIODONTAL DISEASES MAY INFLUENCE DIABETES
Periodontal diseases may induce or perpetuate an elevated systemic chronic inflammatory state.120 Acute bacterial and viral infections are known to increase insulin resistance in people without diabetes, a condition which often persists for weeks to onths
after clinical recovery from the illness.121,122 Such illnesses and resultant increases in insulin resistance in people with diabetes greatly aggravate glycemic control.Chronic Gram-negative periodontal infections may also result in increased insulin resistance and
poor glycemic control.123 Treatment that reduces periodontal inflammation may restore insulin sensitivity, resulting in improved metabolic control. The previously discussed intervention studies that showed improved glycemic control following periodontal therapy support such a hypothesis. Studies suggest that periodontitis patients, particularly those colonized by Gram-negative organisms such as P. gingivalis, Tannerella forsythensis, and Prevotella intermedia, have significantly higher serum markers of inflammation such as C-reactive protein (CRP), IL-6, and fibrinogen than subjects without periodontitis. 124-126 Systemic dissemination of these organisms or their products may induce a acteremia or endotoxemia, inducing an elevated inflammatory state and stimulating increased levels of serum inflammatory markers. In one study, the simple act of chewing caused systemic endotoxemia in 40% of subjects with periodontitis compared to only 12% of periodontally healthy subjects; additionally, the concentration of endotoxin in the bloodstream was fivefold higher in those with periodontitis.127 Periodontal treatment not only reduces clinically evident inflammation, but may also result in decreased erumlevels of IL-6 and CRP.128 This evidence suggests that periodontal diseases have systemic effects that extend beyond the local periodontal environment. The potential impact of elevated systemic proinflammatory mediators in subjects with diabetes is tremendous. Systemic inflammation is significantly elevated in the presence of obesity, insulin resistance, hyperglycemia, and diabetes.1 High serum levels of the acute-phase reactants CRP and fibrinogen are seen in people with insulin resistance and obesity. 9,129 Insulin resistance and obesity are recognized as chronic inflammatory states and share many of the pathophysiologic features of atherosclerosis.9,99 Obesity, atherosclerosis, and insulin resistance are strongly linked to the actions of the proinflammatory cytokines IL-6 and TNF-a and their resultant stimulation of acute phase reactant production in the liver.130 The hallmark of type 2 diabetes is an increase in insulin resistance, which is also strongly linked to obesity.7 Obesity alters the normal metabolic and endocrine function of adipose tissue, resulting in increased production
of fatty acids, hormones, cytokines, and acute phase reactants.131 Adipose tissue has amajor endocrine function, producing a wide array of hormones commonly called ‘‘adipokines.’’132 Changes in bodyfat content result in alterations of adipokine production
and function. These hormones, including leptin, resistin, and adiponectin, among others, participate in regulation of appetite, energy use, insulin sensitivity, blood pressure, angiogenesis, and immune function. 132 An increased body mass index is associated with an increase in the number and size of adipocytes, which are cells with high metabolic activity that produce large quantities of TNF-a and IL-6. In fact, adipose tissue produces about one-third of the total circulating serum level of IL-6.133 Although the exact physiologic pathways have not been fully delineated, obesity may increase insulin resistance by causing elevated production of TNF-a and IL-6 and decreased production of adiponectin.9,134 TNF-a can induce insulin resistance at the receptor level by preventing autophosphorylation of the insulin receptor and suppressing
second messenger signaling through the inhibition of the enzyme tyrosine kinase.131 Infusion of TNF-a in healthy humans directly induces insulin resistance in skeletal muscle and reduces glucose uptake and use.135 Blocking TNF-a with pharmacologic
agents has been shown to reduce seruminsulin levels and improve insulin sensitivity in some subjects136 but not in others.137 Adiponectin antagonizes many of the effects of TNF-a and improves insulin sensitivity. 138 As body mass increases, adiponectin production decreases; thus, obesity results in elevatedTNF-a levels and decreased adiponectic levels, both of which result in insulin resistance.138 IL-6 stimulates TNF-a
production; therefore, increased production of IL-6 from adipocytes in obese individuals causes elevated TNF-a production, which may further exacerbate insulin resistance. The increased production of TNF-a and IL-6 also stimulates greater hepatic CRP production,
which may also increase insulin resistance.9,139 Multiple mechanisms are involved in regulation of insulin sensitivity and resistance, including J Periodontol • August 2006 Mealey, Oates 1297 adipokines,genetic factors,environmentalstresses,and inflammatory mediators. As an inflammatory condition, periodontal diseases may also play a role in this process. Elevated circulating levels of several proinflammatory cytokines have been found in individuals with periodontitis.124-126 Obesity has been associated with an increased risk of periodontal disease.140-142 Compared to subjects with a BMI £20 kg/m2, the risk of periodontitis increased three-fold in Japanese subjects with a BMI between 25 and 30 kg/m2 and over eight-fold in subjects with a BMI ‡30 kg/m.2 The relationship between obesity and periodontitis may be mediated by insulin resistance.140 A recent examination of Third National Health and Nutrition Examination
Survey (NHANES III) data for non-diabetic subjects revealed a positive association between BMI and clinical attachment loss.140 Interestingly, overweight individuals (BMI ‡27) with elevated insulin resistance had a significant odds ratio of 1.48 for severe
periodontal disease compared to overweight individuals without insulin resistance. Subjects in the highest quartile of body mass (BMI ‡30.8 kg/m2) also showed significantly elevated serum levels of TNF-a and solubleTNF-a receptors compared to those in the lowest quartile of body mass, with a BMI <24.6 kg/m.2 These data suggest that increased BMI is associated with both systemic inflammation and periodontal disease. In addition to the elevated systemic inflammatory state associated with obesity and insulin resistance, people with diabetes often have a shift in monocyte/ macrophage phenotype, which results in the overproduction of these same inflammatory cytokines in response to periodontal pathogens.55 Diabetic patients who also have periodontitis may present with an even greater systemic inflammatory condition with elevated serumlevels of IL-6, TNF-a, and CRP, which can worsen insulin resistance and thereby aggravate glycemic control. This could explain why periodontitis
increases the risk of poor glycemic control in patients with type 2 diabetes.105 It may also explain why improvement in glycemic control has followed periodontal therapy in some studies of diabetic subjects.110-112,116,117 In a small study of 13 type 2 diabetic subjects with periodontitis, periodontal treatment consisting of mechanical debridement and local delivery of minocycline resulted in a significant reduction in serumTNF-a levels that was accompanied by a significant reduction in mean HbA1c levels from 8.0%
to 7.1%.143 Reductions in HbA1c values were strongly correlated with the reductions in serum TNF-a levels across the patient population. Thus, periodontal treatment may reduce inflammation locally and also decrease serum levels of the inflammatory ediators
that cause insulin resistance, thereby positively affecting glycemic control.
EFFECTS OF DIABETES ON THE RESPONSE TO PERIODONTAL THERAPY
Only limited evidence is available to evaluate the comparative response to periodontal therapy in diabetic and non-diabetic patients with periodontitis. In well-controlled diabetic subjects, the clinical and microbiologic response to scalingand root planing appears similar to that in non-diabetic individuals.115,144 Although many diabetic patients show improvement in clinical parameters of disease immediately after therapy, patients with poorer glycemic control may have a more rapid recurrence of deep pockets and a less favorable long-term response.145 In one longitudinal study, 20 diabetic and 20 non-diabetic subjects received scaling and root planing, modified Widman flap surgery at sites with residual probing depths ‡5 mm, and regular maintenance therapy.146 Five years after the baseline examination, diabetic and nondiabetic
subjects had a similar percentage of sites demonstrating gain, loss, or no change in clinical attachment. The HbA1c values revealed that most of the diabetic subjects in this study were well controlled or moderately controlled at baseline.146 Further longitudinal
studies of various periodontal treatment modalities are needed to determine the healing response in individuals with diabetes compared to individuals without diabetes.
CONCLUSIONS
Periodontal diseases and diabetes mellitus are closely associated and are highly prevalent chronic diseases with many similarities in pathobiology. Related antecedent
conditions including obesity and insulin resistance may play an important role in this relationship. Inflammation is a critical player in the association, and its importance is just now coming to light. Diabetes clearly increases the risk of periodontal diseases, and biologically plausible mechanisms have been demonstrated in abundance. Less clear is the impact of periodontal diseases on glycemic control of diabetes and the mechanisms through which this occurs. It is possible that periodontal diseases may serve as initiators or propagators of insulin resistance in a way similar to obesity, thereby aggravating glycemic control. Further research is needed to clarify this aspect of the relationship between periodontal diseases and diabetes.
REFERENCES
1. Mealey BL, Ocampo GL. Diabetes mellitus. Periodontol 2000 2006; in press.
2. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Position statement. Diabetes Care 2005;29(Suppl. 1):S37-S42.
3. Mokdad AH, Ford ES, Bowman BA, et al. The continuing increase of diabetes in the U.S. Diabetes Care 2001;24:412. Diabetes Mellitus and Periodontal Diseases Volume 77 • Number 8 1298
4. National Center for Health Statistics. United States, 2005. Chartbook on Trends in the Health of Americans, Table 55. Hyattsville, MD: National Center for Health Statistics; 2005.
5. Centers for Disease Control and Prevention. National Diabetes Fact Sheet. Available at: http://www.cdc.gov/ diabetes/pubs/estimates.htm. Accessed December 27, 2005.
6. Laakso M, Pyorala K. Age at onset and type of diabetes. Diabetes Care 1985;8:114 117.
7. DeFronzo RA, Ferrannini E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia and atherosclerotic cardiovascular disease. Diabetes Care 1991;14:173-194.
8. Rhodes CJ. Type 2 diabetes A matter of b-cell life and death? Science 2005;307:380-384.
9. Festa A, D’Agostino R Jr., Howard G, Mykkanen L, Tracy RP, Haffner SM. Chronic subclinical inflammation as part of the insulin resistance syndrome: The Insulin Resistance Atherosclerosis Study (IRAS). Circulation 2000;102:42-47.
10. Bergman RN, Ader M. Free fatty acids and pathogenesis of type 2 diabetes mellitus. Trends Endocrinol Metab 2000;11:351-356.
11. Saydah SH, Chandra A, Eberhardt MS. Pregnancy experience among women with and without gestational diabetes in the U.S. 1995 national survey of family growth. Diabetes Care 2005;28:1035-1040.
12. Kirwan JP, Varastehpour A, Jing M, et al. Reversal of insulin resistance postpartum is linked to enhanced skeletal muscle insulin signaling. J Clin Endocrinol
Metab 2004;89:4678-4684.
13. DECODE Study Group. Glucose tolerance and mortality:Comparison of WHO and American Diabetes Association diagnostic criteria. European Diabetes Epidemiology Group. Diabetes Epidemiology: Collaborative analysis of diagnostic criteria in Europe.
Lancet 1999;354:617-621.
14. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998;15:539-553.
15. Virtue MA, Furne JK, Nuttall FQ, Levitt MD. Relationship between GHb concentration and erythrocyte survival determined from breath carbon monoxide concentration. Diabetes Care 2004;27:931-935.
16. Rohlfing CL, Wiedmeyer HM, Little RR, England JD, Tennill A, Goldstein DE. Defining the relationshipbetween plasma glucose and HbA1c: Analysis of glucose
profiles and HbA1c in the Diabetes Control and Complications Trial. Diabetes Care 2002;25:275-278.
17. Davidson MB, Schriger DL, Peters AL, Lorber B. Glycosylated hemoglobin as a diagnostic test for type 2 diabetes mellitus. JAMA 2000;283:606-607.
18. Papapanou PN. Periodontal diseases: Epidemiology. Ann Periodontol 1996;1:1-36.
19. Mealey BL, Moritz AJ. Hormonal influences: Effects of diabetes mellitus and endogenous female sex steroid hormones on the periodontium. Periodontol
2000 2003;32:59-81.
20. Sbordone L, Ramaglia L, Barone A, Ciaglia RN, Iacono VJ. Periodontal status and subgingival microbiota of insulin-dependent juvenile diabetics: A 3-year longitudinal
study. J Periodontol 1998;69:120-128.
21. Cianciola L, Park B, Bruck E, Mosovich L, Genco R.Prevalence of periodontal disease in insulin-dependent diabetes mellitus (juvenile diabetes). J Am Dent
Assoc 1982;104:653-660.
22. de Pommereau V, Dargent-Pare C, Robert JJ, Brion M. Periodontal status in insulin-dependent diabetic adolescents. J Clin Periodontol 1992;19:628-632.
23. Gusberti F, Syed S, Bacon G, Grossman N, Loesche W. Puberty gingivitis in insulin-dependent diabetic children. I. Cross-sectional observations. J Periodontol 1983;54:714-720.
24. Sastrowijoto S, van der Velden U, van Steenbergen T, et al. Improved metabolic control, clinical periodontal status, and subgingival microbiology in insulindependent
diabetes mellitus. A prospective study. J Clin Periodontol 1990;17:233-242.
25. Karjalainen K, Knuuttila M. The onset of diabetes and poor metabolic control increases gingival bleeding in children and adolescents with insulin-dependent
diabetes mellitus. J Clin Periodontol 1996;23:1060-1067.
26. Ervasti T, Knuuttila M, Pohjamo L, Haukipuro K. Relation between control of diabetes and gingival bleeding. J Periodontol 1985;56:154-157.
27. Cutler CW, Machen RL, Jotwani R, Iacopino AM. Heightened gingival inflammation and attachment loss in type 2 diabetics with hyperlipidemia. J Periodontol1999;70:1313-1321.
28. Salvi GE, Kandylaki M, Troendle A, Persson GR, Lang NP. Experimental gingivitis in type 1 diabetics: A controlled clinical and microbiological study. J Clin Periodontol 2005;32:310-316.
29. Bacic M, Plancak D, Granic M. CPITN assessment of periodontal status in diabetic patients. J Periodontol 1988;59:816-822.
30. Emrich LJ, Shlossman M, Genco RJ. Periodontal disease in non-insulin dependent diabetes mellitus. J Periodontol 1991;62:123-131.
31. Shlossman M, Knowler WC, Pettitt DJ, Genco RJ.Type 2 diabetes mellitus and periodontal disease. J Am Dent Assoc 1990;121:532-536.
32. Tervonen T, Oliver R. Long-term control of diabetes mellitus and periodontitis. J Clin Periodontol 1993; 20:431-435.
33. Bridges RB, Anderson JW, Saxe SR, Gregory K, Bridges SR. Periodontal status of diabetic and nondiabetic men: Effects of smoking, glycemic control,and socioeconomic factors. J Periodontol 1996;67:1185-1192.
34. Collin HL, Uusitupa M, Niskanen L, et al. Periodontal findings in elderly patients with non-insulin dependent diabetes mellitus. J Periodontol 1998;69:962-966.
35. Moore PA, Weyant RJ, Mongelluzzo MB, et al. Type 1 diabetes mellitus and oral health: Assessment of periodontal disease. J Periodontol 1999;70:409-417.
36. Tervonen T, Karjalainen K, Knuuttila M, Huumonen S. Alveolar bone loss in type 1 diabetic subjects. J Clin Periodontol 2000;27:567-571.
37. Campus G, Salem A, Uzzau S, Baldoni E, Tonolo G.Diabetes and periodontal disease: A case-control study. J Periodontol 2005;76:418-425.
38. Nelson RG, Shlossman M, Budding LM, et al. Periodontal disease and NIDDM in Pima Indians. Diabetes Care 1990;13:836-840.
39. Taylor GW, Burt BA, Becker MP, et al. Non-insulin dependent diabetes mellitus and alveolar bone loss progression over 2 years. J Periodontol 1998;69:76-83. J Periodontol • August 2006 Mealey, Oates 1299
40. Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977-986.
41. U.K. Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837-853.
42. Tsai C, Hayes C, Taylor GW. Glycemic control of type 2 diabetes and severe periodontal disease in the U.S. adult population. Community Dent Oral Epidemiol
2002;30:182-192.
43. Safkan-Seppala B, Ainamo J. Periodontal conditions in insulin-dependent diabetes mellitus. J Clin Periodontol 1992;19:24-29.
44. Tervonen T, Knuuttila M. Relation of diabetes control to periodontal pocketing and alveolar bone level. Oral Surg Oral Med Oral Pathol 1986;61:346-349.
45. Guzman S, Karima M, Wang H-Y, Van Dyke TE.Association between interleukin-1 genotype and periodontal disease in a diabetic population. J Periodontol 2003;74:1183-1190.
46. Seppala B, Seppala M, Ainamo J.Alongitudinal study on insulin-dependent diabetes mellitus and periodontal disease. J Clin Periodontol 1993;20:161-165.
47. Seppala B, Ainamo J. A site-by-site follow-up study on the effect of controlled versus poorly controlled insulin-dependent diabetes mellitus. J Clin Periodontol 1994;21:161-165.
48. Barnett M, Baker R, Yancey J, MacMillan D, Kotoyan M. Absence of periodontitis in a population of insulindependent diabetes mellitus patients. J Periodontol 1984;55:402-405.
49. Sastrowijoto S, Hillemans P, van Steenbergen T, Abraham-Inpijn L, de Graaff J. Periodontal condition and microbiology of healthy and diseased periodontal pockets in type 1 diabetes mellitus patients. J Clin Periodontol 1989;16:316-322.
50. Lo¨e H. Periodontal disease. The sixth complication of diabetes mellitus. Diabetes Care 1993;16(Suppl. 1): 329-334.
51. American Academy of Periodontology. Diabetes and periodontal diseases (position paper). J Periodontol 1999;70:935-949.
52. Zambon JJ, Reynolds H, Fisher JG, Shlossman M, Dunford R, Genco RJ. Microbiological and immunological studies of adult periodontitis in patients with
non-insulin dependent diabetes mellitus. J Periodontol 1988;59:23-31.
53. Manouchehr-Pour M, Spagnuolo PJ, Rodman HM, Bissada NF. Comparison of neutrophil chemotactic response in diabetic patients with mild and severe periodontal disease. J Periodontol 1981;52:410-415.
54. McMullen JA, Van Dyke TE, Horoszewicz HU, Genco RJ.Neutrophil chemotaxis in individuals with advanced periodontal disease and a genetic predisposition todiabetes mellitus. J Periodontol 1981;52:167-173.
55. Salvi GE, Collins JG, Yalda B, Arnold RR, Lang NP, Offenbacher S. Monocytic TNF-a secretion patterns in IDDM patients with periodontal diseases. J Clin Periodontol 1997;24:8-16.
56. Naguib G, Al-Mashat H, Desta T, Graves D. Diabetes prolongs the inflammatory response to a bacterial stimulus through cytokine dysregulation. J Invest Dermatol 2004;123:87-92.
57. Salvi GE, Yalda B, Collins JG, et al. Inflammatory mediator response as a potential risk marker for periodontal diseases in insulin-dependent diabetes mellitus patients. J Periodontol 1997;68:127-135.
58. Engebretson SP, Hey-Hadavi J, Ehrhardt FJ, et al. Gingival crevicular fluid levels of interleukin-1b and glycemic control in patients with chronic periodontitis and type 2 diabetes. J Periodontol 2004;75:1203-1208.
59. Loder RT. The influence of diabetes mellitus on the healing of closed fractures. Clin Orthop 1988;232:210-216.
60. White CB, Turner NS, Lee GC, Haidukewych GJ.Open ankle fractures in patients with diabetes mellitus.Clin Orthop 2003;414:37-44.
61. Stuart MJ, Morrey BF. Arthrodesis of the diabetic neuropathic ankle joint. Clin Orthop 1990;253:209-211.
62. Inaba M, Nishizawa Y, Mita K, et al. Poor glycemic control impairs the response of biochemical parameters of bone formation and resorption to exogenous 1,25-dihydroxyvitamin D3 in patients with type 2 diabetes. Osteoporos Int 1999;9:525-531.
63. Tisdel CL, Marcus RE, Heiple KG. Triple arthrodesis for diabetic peritalar neuroarthropathy. Foot Ankle Int 1995;16:332-338.
64. Beam HA, Parsons JR, Lin SS. The effects of blood glucose control upon fracture healing in the BB Wistar rat with diabetes mellitus. J Orthop Res 2002;20:1210-1216.
65. Gooch HL, Hale JE, Fujioka H, Balian G, Hurwitz SR. Alterations of cartilage and collagen expression during fracture healing in experimental diabetes. Connect Tissue Res 2000;41:81-85.
66. Lu H, Kraut D, Gerstenfeld LC, Graves DT. Diabetes interferes with bone formation by affecting the expression of transcription factors that regulate osteoblast differentiation. Endocrinology 2003;144:346-352.
67. Amir G, Rosenmann E, Sherman Y, Greenfeld Z, Ne’eman Z, Cohen AM. Osteoporosis in the Cohen diabetic rat: Correlation between histomorphometric changes in bone and microangiopathy. Lab Invest 2002;82:1399-1405.
68. He H, Liu R, Desta T, Leone C, Gerstenfeld L, Graves D. Diabetes causes decreased osteoclastogenesis, reduced bone formation, and enhanced apoptosis of osteoblastic cells in bacteria stimulated bone loss.Endocrinology 2004;145:447-452.
69. Liu R, Desta T, He H, Graves D. Diabetes alters the response to bacteria by enhancing fibroblast apoptosis. Endocrinology 2004;145:2997-3003.
70. Liu R, Bal HS, Desta T, Krothapalli N, Alyassi M,Luan Q, Graves DT. Diabetes enhances periodontal bone loss through enhanced resorption and diminished bone formation. J Dent Res 2006;85:510-514.
71. Ficara AJ, Levin MP, Grower MF, Kramer GD. A comparison of the glucose and protein content of gingival crevicular fluid from diabetics and nondiabetics. J Periodontal Res 1975;10:171-175.
72. Nishimura F, Takahashi K, Kurihara M, Takashiba S, Murayama Y. Periodontal disease as a complication of diabetes mellitus. Ann Periodontol 1998;3:20-29.
73. Wautier J-L, Guillausseau P-J. Diabetes, advanced glycation endproducts and vascular disease. Vasc Med 1998;3:131-137.
74. Frantzis TG, Reeve CM, Brown AL. The ultrastructure of capillary basement membranes in the attached Diabetes Mellitus and Periodontal Diseases Volume 77 • Number 8 1300 gingiva of diabetic and non-diabetic patients with periodontal disease. J Periodontol 1971;42:406-411.
75. Seppala B, Sorsa T, Ainamo J. Morphometric analysis of cellular and vascular changes in gingival connective tissue in long-term insulin-dependent diabetes. J Periodontol 1997;68:1237-1245.
76. Monnier VM, Glomb M, Elgawish A, Sell DR. The mechanism of collagen cross-linking in diabetes. A puzzle nearing resolution. Diabetes 1996;45(Suppl.3):S67-S72.
77. Schmidt AM, Weidman E, Lalla E, et al. Advanced glycation endproducts (AGEs) induce oxidant stress in the gingiva: A potential mechanism underlying accelerated periodontal disease associated with diabetes.J Periodontal Res 1996;31:508-515.
78. Schmidt AM, Yan SD, Wautier J-L, Stern D. Activation of receptor for advanced glycation end products. A mechanism for chronic vascular dysfunction in diabetic
vasculopathy and atherosclerosis. Circ Res 1999;84:489-497.
79. Paques M, Massin P, GaudricA.Growth factors and diabetic retinopathy. Diabetes Metab 1997;23:125-130.
80. Chiarelli F, Santilli F, Mohn A. Role of growth factors in the development of diabetic complications. Horm Res 2000;53:53-67.
81. Unlu F, Gurdal Guneri P, Hekimgil M, Yesilbek B,Boyacioglu H. Expression of vascular endothelial growth factor in human periodontal tissues: Comparison
of healthy and diabetic patients. J Periodontol 2003;74:181-187.
82. Monnier VM, Bautista O, Kenny D, et al. Skin collagen glycation, glycoxidation, and crosslinking are lower in subjects with long-term intensive versus conventional therapy of type 1 diabetes. Diabetes 1999;48:870-880.
83. Odetti P, Traverso N, Cosso L, Noberasco G, Pronzato MA, Marinari UM. Good glycaemic control reduces oxidation and glycation end-products in collagen of diabetic rats. Diabetologia 1996;39:1440-1447.
84. Turk Z, Misur I, Turk N, Benko B. Rat tissue collagen modified by advanced glycation: Correlation with duration of diabetes and glycemic control. Clin Chem
Lab Med 1999;37:813-820.
85. Wang X, Shen X, Li X, Agrawal CM. Age-related changes in the collagen network and toughness of bone. Bone 2002;31:1-7.
86. Vashisht D, Gibson GJ, Khoury JI, Schaffler MB, Kimura J, Fyhrie DP. Influence of nonenzymatic glycation on biomechanical properties of cortical bone. Bone 2001;28:195-201.
87. Verzijl N, DeGroot J, Ben ZC, et al. Crosslinking by advanced glycation end products increases the stiffness of the collagen network in human articular cartilage: A possible mechanism through which age is a risk factor for osteoarthritis. Arthritis Rheum 2002;46:114-123.
88. Gunczler P, Lanes R, Paoli M, Martinis R, Villaroel O, Weisinger JR. Decreased bone mineral density and bone formation markers shortly after diagnosis of clinical type 1 diabetes mellitus. J Pediatr Endocrinol Metab 2001;14:525-528.
89. McCarthy AD, Etcheverry SB, Bruzzone L, Lettierri G, Barrio DA, Cortizo AM. Non-enzymatic glycosylation of a type I collagen matrix: Effects on osteoblastic development and oxidative stress. BMC Cell Biol 2001;2:16-21.
90. Santana RB, Xu L, Chase HB, Amar S, Graves DT, Trackman PC. A role for advanced glycation end products in diminished bone healing in type 1 diabetes.
Diabetes 2003;52:1502-1510.
91. Takagi M, Kasayama S, Yamamoto T, et al. Advanced glycation endproducts stimulate interleukin- 6 production by human bone-derived cells. J Bone Miner Res 1997;12:439-446.
92. Valerio G, Franzese A, Esposito-Del Puente A, et al. Increased urinary excretion of collagen crosslinks in type 1 diabetic children in the first 5 years of disease.Horm Res 1999;51:173-177.
93. Okazaki R, Totsuka Y, Hamano K, et al. Metabolic improvement of poorly controlled noninsulin-dependent diabetes mellitus decreases bone turnover. J Clin Endocrinol Metab 1997;82:2915-2920.
94. Okazaki R, Miura M, Toriumi M, et al. Short-term treatment with troglitazone decreases bone turnover in patients with type 2 diabetes mellitus. Endocr J 1999;46:795-801.
95. Erbagci AB, Araz M, Ergabci A, Tarakcioglu M, Namiduru ES. Serum prolidase activity as a marker of osteoporosis in type 2 diabetes mellitus. Clin Biochem 2002;35:263-268.
96. Cloos C, Wahl P, Hasslacher C, et al. Urinary glycosylated, free and total pyridinolines and free and total deoxypyridinoline in diabetes mellitus. Clin
Endocrinol (Oxf) 1998;48:317-323.
97. Schmidt AM, Hori O, Cao R, et al. RAGE: A novel cellular receptor for advanced glycation end products. Diabetes 1996;45(Suppl. 3):S77-S80.
98. Katz J, Bhattacharyya I, Farkhondeh-Kish F, Perez FM, Caudle RM, Heft MW. Expression of the receptor of advanced glycation end products in gingival tissues
of type 2 diabetes patients with chronic periodontal disease: A study utilizing immunohistochemistry and RT-PCR. J Clin Periodontol 2005;32:40-44.
99. Ross R. Atherosclerosis – An inflammatory disease. N Engl J Med 1999;340:115-126.100. Lalla E, Lamster IB, Feit M, et al. Blockade of RAGE suppresses periodontitis-associated bone loss in diabetic mice. J Clin Invest 2000;105:1117-1124.
101. Willershausen-Zonnchen B, Lemmen C, Hamm G. Influence of high glucose concentrations on glycosaminoglycan and collagen synthesis in cultured
human gingival fibroblasts. J Clin Periodontol 1991;18:190-195.
102. Ramamurthy NS, Zebrowski EJ, Golub LM. Insulin reversal of alloxan-diabetes induced changes in gingival collagen metabolism of the rat. J Periodontal Res 1974;9:199-206.
103. Ryan M, Ramamurthy NS, Sorsa T, Golub LM. MMPmediated events in diabetes. Ann N Y Acad Sci 1999;878:311-334.
104. Sorsa T, Ingman T, Suomalainen K, et al. Cellular source and tetracycline inhibition of gingival crevicular fluid collagenase of patients with labile diabetes mellitus. J Clin Periodontol 1992;19:146-149.
105. Taylor GW, Burt BA, Becker MP, et al. Severe periodontitis and risk for poor glycemic control in patients with non-insulin-dependent diabetes mellitus. J Periodontol 1996;67:1085-1093.
106. Thorstensson H, Kuylensteirna J, Hugoson A. Medical status and complications in relation to periodontal disease experience in insulin-dependent diabetics.J Clin Periodontol 1996;23:194-202.
107. Saremi A, Nelson RG, Tulloch-Reid M, et al. Periodontal disease and mortality in type 2 diabetes. Diabetes Care 2005;28:27-32. J Periodontol • August 2006 Mealey, Oates 1301
108. Williams RC Jr., Mahan CJ. Periodontal disease and diabetes in young adults. JAMA 1960;172:776-778.
109. Golub LM, Lee H-M, Ryan ME. Tetracyclines inhibit connective tissue breakdown by multiple nonantimicrobial mechanisms. Adv Dent Res 1998;12:12-26.
110. Miller LS, Manwell MA, Newbold D, et al. The relationship between reduction in periodontal inflammation and diabetes control: A report of 9 cases. J Periodontol 1992;63:843-848.
111. Grossi SG, Skrepcinski FB, DeCaro T, Zambon JJ, Cummins D, Genco RJ. Response to periodontal therapy in diabetics and smokers. J Periodontol 1996; 67:1094-1102.
112. Grossi SG, Skrepcinski FB, DeCaro T, et al. Treatment of periodontal disease in diabetics reduces glycated hemoglobin. J Periodontol 1997;68:713-719.
113. Promsudthi A, Pimapansri S, Deerochanawong C, Kanchanavasita W. The effect of periodontal therapy on uncontrolled type 2 diabetes mellitus in older subjects. Oral Dis 2005;11:293-298.
114. Aldridge JP, Lester V, Watts TLP, Collins A, Viberti G, Wilson RF. Single-blind studies of the effects of improved periodontal health on metabolic control in Type 1 diabetes mellitus. J Clin Periodontol 1995;22:271-275.
115. Christgau M, Palitzsch KD, Schmalz G, Kreiner U,Frenzel S. Healing response to non-surgical periodontal therapy in patients with diabetes mellitus: Clinical, microbiological, and immunological results. J Clin Periodontol 1998;25:112-124.
116. Stewart JE, Pager KA, Friedlander AH, Zadeh HH. The effect of periodontal treatment on glycemic control in patients with type 2 diabetes mellitus. J Clin
Periodontol 2001;28:306-310.
117. Kiran M, Arpak N, Unsal E, Erdogan MF. The effect of improved periodontal health on metabolic control in type 2 diabetes mellitus. J Clin Periodontol 2005;32:266-272.
118. Rodrigues DC, Tabe MJ, Novaes AB, Souza SL, Grisi MF. Effect of non-surgical periodontal therapy on glycemic control in patients with type 2 diabetes mellitus. J Periodontol 2003;74:1361-1367.
119. Janket S-J, Wightman A, Baird AE, Van Dyke TE, Jones JA. Does periodontal treatment improve glycemic control in diabetic patients? A meta-analysis of intervention studies. J Dent Res 2005;84:1154-1159.
120. Loos BG. Systemic markers of inflammation in periodontitis. J Periodontol 2005;76:2106-2115.
121. Sammalkorpi K. Glucose intolerance in acute infections.J Intern Med 1989;225:15-19.
122. Yki-Jarvinen H, Sammalkorpi K, Koivisto VA, Nikkila EA. Severity, duration and mechanism of insulin resistance during acute infections. J Clin Endocrinol Metab 1989;69:317-323.
123. Genco RJ, Grossi SG, Ho A, Nishimura F, Murayama Y. A proposed model linking inflammation to obesity, diabetes and periodontal infections. J Periodontol 2005;76:2075-2084.
124. Noack B, Genco RJ, Trevisan M, Grossi S, Zambon JJ, De Nardin E. Periodontal infections contribute to elevated systemic C-reactive protein level. J Periodontol2001;72:1221-1227.
125. Loos BG, Craandiji J, Hoek FJ, Wertheim-van Dillen PME, van der Velden U. C-reactive protein and other markers of systemic inflammation in relation to cardiovascular diseases are elevated in periodontitis.J Periodontol 2000;71:1528-1534.
126. Wu T, Trevisan M, Genco RJ, Falkner KL, Dorn JP, Sempos CT. Examination of the relation between periodontal health status and cardiovascular risk factors: Serum total and high density lipoprotein cholesterol, C-reactive protein, and plasma fibrinogen. Am
J Epidemiol 2000;151:273-282.
127. Geerts SO, Nys M, De Mol P, et al. Systemic release of endotoxins induced by gentle mastication: Association with periodontitis severity. J Periodontol 2002;73:73-78.
128. D’Aiuto F, Parker M, Andreou G, et al. Periodontitis and systemic inflammation: Control of the local infection is associated with a reduction in serum inflammatory markers. J Dent Res 2004;83:156-160.
129. Haffner S, Temprosa M, Crandall J, et al. Intensive lifestyle intervention or metformin on inflammation and coagulation in participants with impaired glucose tolerance. Diabetes 2005;54:1566-1572.
130. Pickup JC, Crook MA. Is type 2 diabetes mellitus a disease of the innate immune system? Diabetologia 1998;41:1241-1248.
131. Fernandez-Real JM, Ricart W. Insulin resistance and chronic cardiovascular inflammatory syndrome.Endocr Rev 2003;24:278-301.
132. Ronti T, Lupattelli G, Mannarino E. The endocrine function of adipose tissue: An update. Clin Endocrinol (Oxf) 2006;64:355-365.
133. Mohamed-Ali V, Goodrick S, Rawesh A, et al. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-a, in vivo. J Clin Endocrinol
Metab 1997;82:4196-4200.
134. Kadowaki T, Yamauchi T. Adiponectin and adiponectine receptors. Endocr Rev 2005;26:439-451.
135. Plomgaard P, Bouzakri K, Krogh-Madsen R, Mittendorfer B, Zierath JR, Pedersen BK. Tumor necrosis factor-alpha induces skeletal muscle insulin resistance in healthy human subjects via inhibition of Akt substrate 160 phosphorylation. Diabetes 2005;54:
2939-2945.
136. Gonzalez-Gay MA, DeMatias JM, Gonzalez-Juanatey C, et al. Anti-tumor necrosis-alpha blockade improves insulin resistance in patients with rheumatoid arthritis. Clin Exp Rheumatol 2006;24:83-86.
137. Dominguez H, Storgaard H, Rask-Madsen C, et al. Metabolic and vascular effects of tumor necrosis factor-alpha blockade with etanercept in obese patients with type 2 diabetes. J Vasc Res 2005;42:517-525.
138. Whitehead JP, Richards AA, Hickman IJ, Macdonald GA, Prins JB. Adiponectin – A key adipokine in the metabolic syndrome. Diabetes Obes Metab 2006;8:264-280.
139. Natali A, Toschi E, Baldeweg S, et al. Clustering of insulin resistance with vascular dysfunction and low-grade inflammation in type 2 diabetes. Diabetes 2006;55:1133-1140.
140. Saito T, Shimazaki Y, Sakamoto M. Obesity and periodontitis. N Engl J Med 1998;339:482-483.
141. Wood N, Johnson RB, Streckfus CF. Comparison of body composition and periodontal disease using nutritional assessment techniques: Third National Health and Nutrition Examination Survey (NHANES III). J Clin Periodontol 2003;30:321-327.
142. Nishida N, Tanaka M, Hayashi N, et al. Determination of smoking and obesity as periodontitis risks Diabetes Mellitus and Periodontal Diseases Volume 77 • Number 8
1302 using the classification and regression tree method. J Periodontol 2005;76:923-928.
143. Iwamoto Y, Nishimura F, Nakagawa M, et al. The effects of antimicrobial periodontal treatment on circulating tumor necrosis factor-alpha and glycated hemoglobin level in patients with type 2 diabetes. J Periodontol 2001;72:774-778.
144. Tervonen T, Knuuttila M, Pohjamo L, Nurkkala H. Immediate response to non-surgical periodontal treatment in subjects with diabetes mellitus. J Clin Periodontol 1991;18:65-68.
145. Tervonen T, Karjalainen K. Periodontal disease related to diabetic status. A pilot study of the response to periodontal therapy in type 1 diabetes. J Clin Periodontol 1997;24:505-510.
146. Westfelt E, Rylander H, Blohme G, Jonasson P, Lindhe J. The effect of periodontal therapy in diabetics. Results after 5 years. J Clin Periodontol 1996; 23:92-100.
Correspondence: Dr. Brian L. Mealey,
Department of Periodontics,
University of Texas Health Science Center at San Antonio,
Mail Code 7894,
7703 Floyd Curl Dr., San Antonio, TX 78229.
E-mail: [email protected].
Accepted for publication May 22, 2006.
J Periodontol • August 2006 Mealey, Oates 1303
Researchers report periodontal disease independently predicts new onset diabetes
August 6, 2008 — Periodontal disease may be an independent predictor of incident Type 2 diabetes, according to a study by researchers at Columbia University Mailman School of Public Health. While diabetes has long been believed to be a risk factor for periodontal infections, this is the first study exploring whether the reverse might also be true, that is, if periodontal infections can contribute to the development of diabetes. The full study findings are published in the July 2008 issue of Diabetes Care.
The Mailman School of Public Health researchers studied over 9,000 participants without diabetes from a nationally representative sample of the U.S. population, 817 of whom went on to develop diabetes. They then compared the risk of developing diabetes over the next 20 years between people with varying degrees of periodontal disease and found that individuals with elevated levels of periodontal disease were nearly twice as likely to become diabetic in that 20 year timeframe. These findings remained after extensive multivariable adjustment for potential confounders including, but not limited to, age, smoking, obesity, hypertension, and dietary patterns.
“These data add a new twist to the association and suggest that periodontal disease may be there before diabetes,” said Ryan T. Demmer, PhD, MPH, associate research scientist in the Department of Epidemiology at the Mailman School of Public Health and lead author. “We found that over two decades of follow-up, individuals who had periodontal disease were more likely to develop Type 2 diabetes later in life when compared to individuals without periodontal disease.”
Also of interest, the researchers found that those study participants who had lost all of their teeth were at intermediate risk for incident diabetes. “This could be suggestive that the people who lost all of their teeth had a history of infection at some point, but subsequently lost their teeth and removed the source of infection,” noted Dr. Demmer. “This is particularly interesting as it supports previous research originating from The Oral Infections and Vascular Disease Epidemiology Study (INVEST) which has shown that individuals lacking teeth are at intermediate risk for cardiovascular disease” said Moïse Desvarieux, MD, PhD, director of INVEST, associate professor and Inserm Chair of Excellence in the Department of Epidemiology at the Mailman School and senior author of the paper.
The contributory role of periodontal disease in the development of Type 2 diabetes is potentially of public health importance because of the prevalence of treatable periodontal diseases in the population and the pervasiveness of diabetes-associated morbidity and mortality. However, observes Dr. Demmer, more studies are needed both to determine whether gum disease directly contributes to type 2 diabetes and, from there, that treating the dental problem can prevent diabetes. In addition to Dr. Desvarieux, David R. Jacobs Jr., PhD, professor in the Department of Epidemiology and Community Health at the University of Minnesota, also contributed to the research.
In the recent issue of “DIABETES CARE” , February 2004, page 615, Letters and Observations, Japanese researchers investigating the relationship between periodontal disease and diabetic retinopathy, stated the following, “the severity of periodontal disease was significantly correlated with the severity of diabetic retinopathy, and the risk of proliferative diabetic retinopathy wsa significantly higher in the presence of periodontal disease. There was no significant relationship between the severity of periodontal disease and HbA1c, or duration of diabetes. There was a significant relationship between the severity of diabetic retinopathy and duration of diabetes…”
Bacteria from Gum Infections Associated with Diabetes, Chronic Lung Disease
VANCOUVER, British Columbia — Diabetes and chronic lung disease can be added to the growing list of systemic diseases and conditions associated with bacteria from infected gums, new studies from the University at Buffalo School of Dental Medicine have shown. The findings from both studies were presented here today (March 13) at the combined meeting of the American Association of Dental Research and International Association of Dental Research.
To investigate the association of periodontal disease with diabetes, a research team headed by Sara G. Grossi, D.D.S., UB senior research scientist, concentrated on insulin resistance, a known precursor of active diabetes, in which cells do not absorb insulin from the blood stream. As their study group, the researchers used 11,198 subjects from the Third National Health and Nutrition Examination Survey (NHANES III) conducted from 1988-94, including all non-diabetic NHANES participants between the ages of 20 and 90 who had at least six natural teeth. They assessed information on periodontal status, defined as degree of gum detachment from bone, along with fasting-insulin and fasting-glucose levels, which were combined to establish an index of insulin resistance. Persons with known diabetes or with a blood glucose level that reached diabetic levels were excluded from the analysis.
Analysis showed that the index of insulin resistance increased as severity of periodontal disease increased. The relationship was not affected by age, gender, body-mass index (a measure of obesity) or smoking. To clarify the relationship further, the researchers separated the study group into overweight versus non-overweight, using a body mass index of 27 as the dividing line. Weight is an independent risk factor for insulin resistance and diabetes.
Results showed that those with severe periodontal disease (gum detachment), regardless of weight, have a higher index of insulin resistance than those with little or no disease. “Gram-negative periodontal infections are significantly associated with insulin resistance in non-diabetics,” Grossi said. “We know that when diabetics have an acute infection, their diabetes goes out of control. Gram-negative bacteria produce a very potent toxin called LPS, which probably interferes with the action of insulin and is responsible for maintaining a chronic state of insulin resistance in people with gum infections,” she said.
The study on the relationship between periodontal infection and chronic lung disease was designed to follow up earlier reports of a link between poor oral hygiene, gum disease and chronic lung disease, also using data from NHANES III. Frank Scannapieco, D.M.D, Ph.D., assistant professor of oral biology, analyzed data from 13,792 adults concerning the incidence of pneumonia, asthma, bronchitis and/or emphysema and the condition of their oral health, using degree of gum detachment from bone as an indicator.
Results showed that persons with chronic lung conditions had more gum detachment than those with no lung disease, after correction for age, gender, race, ethnicity, education, income, frequency of dental visits, smoking and alcohol consumption. There also was a direct correlation between the amount of detachment and lung-disease risk. Subjects with gum detachment that exceeded 2 mm had a 40 percent greater risk of developing lung disease than those with attachment loss of less than 2 mm, results showed.
http://www.newswise.com/articles/view/?id=PERIODIS.UBU
Chronic Periodontal Disease Could Lead To Diabetes
ScienceDaily (Apr. 23, 2001) — BETHESDA, Md. – Chronic periodontal disease may contribute to diabetes, according to a review of recent research presented today. While it has been established that people with diabetes are more prone to developing periodontal disease, new research is suggesting that periodontal disease may, in turn, be a risk factor for diabetes.
The research review was presented at an American Academy of Periodontology (AAP)/National Institute of Dental and Craniofacial Research (NIDCR) symposium on periodontal systemic connections in Bethesda, Md. Periodontal disease can cause bacteria to enter the bloodstream and activate immune cells. These activated cells produce inflammatory biological signals (cytokines) that have a destructive effect throughout the entire body. “In the pancreas, the cells responsible for insulin production can be damaged or destroyed by the chronic high levels of cytokines. Once this happens, it may induce Type 2 diabetes, even in otherwise healthy individuals with no other risk factors for diabetes,” explained presenter Anthony Iacopino, D.M.D., Ph.D. in the Division of Prosthodontics at Marquette University’s School of Dentistry in Milwaukee, Wis.
According to Iacopino, hyperlipidemia or high serum cholesterol, not impaired glucose tolerance, seems to be a significant risk factor for periodontal disease in diabetics. “Therefore, lipid-lowering therapies, such as low-fat diets, lipid lowering drugs and exercise, are vitally important for diabetics who want to improve their quality of life, as well as their oral health,” he said. “The same approaches may also prove beneficial in non-diabetic patients with high cholesterol.”
The next step to determine for sure whether or not periodontal disease can cause diabetes is to perform clinical studies and intervention trials, which answer the question, when periodontal disease is treated, does the risk for diabetes decrease? “Until we have results from intervention studies to better understand the role periodontal disease may play in diabetes, as well as heart disease, preterm births and respiratory disease, the best advice is for people to take excellent care of their oral health to help ensure they keep their teeth as well as maintain overall health,” said Michael McGuire, D.D.S., president of the AAP.
Clinical Diabetes 23:171-178, 2005
© American Diabetes Association ®, Inc., 2005
Diabetes and Periodontal Infection: Making the Connection
Janet H. Southerland, DDS, MPH, PhD, George W. Taylor, DMD, DrPH and Steven Offenbacher, DDS, PhD, MMSc
The oral cavity provides a continuous source of infectious agents, and its condition often reflects progression of systemic pathologies. Historically, oral infections were thought to be localized to the oral cavity except in the case of some associated syndromes and untreated odontogenic abcesses. A change in paradigm has dispelled this notion, and a whole new concept of the status of the oral cavity and its impact on systemic health and disease has evolved.
Diabetes affects > 18 million individuals in the United States and > 171 million individuals worldwide and has reached epidemic status.1 The disease is characterized by an increased susceptibility to infection, poor wound healing, and increased morbidity and mortality associated with disease progression. Diabetes is also recognized as an important risk factor for more severe and progressive periodontitis, infection or lesions resulting in the destruction of tissues and supporting bone that form the attachment around the tooth.
Both diseases are thought to share a common pathogenesis that involves an enhanced inflammatory response that can be observed at the local and systemic level.2-6 The inflammatory response is mainly caused by the chronic effects of hyperglycemia and specifically the formation of biologically active glycated proteins and lipids that promote inflammatory responses.7,8
Although there are undoubtedly underlying genetic contributions to diabetes and periodontitis, the focus of research in this area has targeted the bacterial and host contributions to expression of disease.9-11 Both populationbased and mechanistic studies have examined the potientating effects of periodontal infection in the presence of hyperglycemia and have demonstrated increased innate immune responses and periodontal tissue destruction related to an altered inflammatory response.12-15 Collectively these studies have provided insight into molecular mechanisms that support observed epidemiological associations between periodontal diseases and diabetes. The purpose of this review is to make the connection between periodontal disease and diabetes based on information in the literature and to discuss proper management and referral of patients who have signs and symptoms of periodontal disease and other oral complications.
PERIODONTAL DISEASE AND BACTERIAL INFECTION
Periodontal infection represents a complication that may be involved in altering systemic physiology in diabetic patients. Since periodontitis can be more than just a localized oral infection, the effects have been hypothesized to be far-reaching.16,17 Severe chronic forms of this disease can result in systemic response to the bacteria and bacterial products that are disseminated due to breakdown of the periodontal apparatus (the ligament attachment around the tooth that includes the gingival tissues and bone). The interrelationships between diabetes and periodontal disease provide an example of systemic disease predisposing to oral infection, and once that infection is established, the oral infection exacerbates the progression of systemic disease.
In addition, it is also possible for oral infection to serve as a metabolic stressor that may exacerbate systemic disease. In order to understand cellular and molecular mechanisms responsible for such a cyclical association, one must identify common physiological changes associated with diabetes and periodontitis that produce a cooperative effect when the conditions coexist. Accumulation of advanced glycation end products (AGEs) as a result of the chronic hyperglycemic state or diabetes, coupled with the presence of infection and an exaggerated host response, may provide a viable explanation for the clinical outcomes observed in diabetic patients with periodontal disease.
Bacterial products such as endotoxin or lipopolysaccharide (LPS) also play a role in propagating an inflammatory response in the host through the Toll-like protein receptors (TLRs) and thus can induce an inflammatory cascade.18 These receptors play an important role in the innate immune response, particularly in the initial interaction between the infecting microorganisms, such as Porphyromonas gingivalis, and phagocytic cells of the monocyte lineage.19 Genetic and biochemical studies have shown that the toll protein family members play a critical role in the immediate response to infection.20,21 Although LPS monocyte interactions provide one of the best-studied models of innate immunity using gram-negative bacteria and the bacterial endotoxin, the mechanisms behind periodontal disease and the regulation of TLR protein expression are still not well understood.
Chronic hyperglycemia has been closely associated with an inflammatory response that has been linked to complications observed in diabetes. The presence of periodontal disease represents a unique opportunity for oral pathogens and their products to gain access to the systemic circulation. Bacterial toxins are known to elicit immune responses that can disrupt homeostasis of the system and in some instances can result in lethal outcomes to the individual.
Diabetes and periodontal disease are common chronic diseases observed in the U.S. population. These diseases are thought to be associated biologically, and a number of reviews and studies have proposed mechanisms to explain the relationship, including 1) microvascular disease, 2) changes in components of gingival crevicular fluid, 3) changes in collagen metabolism, 4) an altered host response, 5) altered subgingival flora, 6) genetic predisposition, and 7) nonenzymatic glycation.22-28
In addition, in vitro studies of monocytes from people with diabetes have shown a hyperresponsive phenotype with overexpression of pro-inflammatory mediators such as interleukin-1ß (IL-1ß), tumor necrosis factor- (TNF-), and prostaglandin E2.3,29 In similar in vivo studies, patients with periodontitis and diabetes were found to have significantly higher levels of local inflammatory mediators compared to systemically healthy individuals with periodontal disease.13,30
Advances in the molecular biology of insulin resistance and ß-cell dysfunction increasingly support a role for inflammatory mediators, particularly cytokines, and elements of the innate immune system in the pathogenesis of type 2 diabetes.31 Cytokine production as a consequence of an infectious challenge could potentially contribute to insulin resistance in a number of ways, including 1) modification of insulin receptor substrate-1 by serine phosphorylation,32 2) alteration of adipocyte function with increased production of free fatty acids,33,34 and 3) diminution of endothelial nitric oxide production.35,36 The process may also alter pancreatic ß-cell function, either acting directly37,38 or through stimulation of free fatty acid production.39,40 In fact, cytokine-induced mechanisms have been suggested to participate in the ß-cell damage or “burn-out” seen in animal obesity models of type 2 diabetes that may be mediated through a c-Jun NH2-terminal kinase-induced insulin resistance model.41-43
Fasting insulin is considered a marker, though imperfect, of insulin resistance. Increased resistance to skeletal muscle glucose uptake is part of the physiological adjustment to the catabolic milieu seen in inflammation.44-47 As cytokines or inflammatory mediators decrease insulin sensitivity, insulin resistance may be part of a causal pathway linking inflammatory mediators to incident diabetes. Adipocytes produce large quantities of cytokines, such as TNF- and IL-1ß, in the presence of inflammation.34 Infections have been investigated with regard to the development of coronary heart disease, with mechanisms similar to those discussed here being proposed to mediate their effects.48-50 Diabetes and infections have long been known to contribute to metabolic dysregulation.51-53 This fact allows us to speculate that repeated or chronic infections such as periodontitis, or some susceptibility to them, may represent an additional causal element for type 2 disease, a hypothesis that deserves further study.
ADVANCED GLYCATION
Evidence has accumulated supporting a role for AGEs in exacerbating diabetic systemic complications and periodontal disease severity associated with a chronic and intense inflammatory response. Moreover, AGEs have been associated with enhanced oxidant stress54,55 and subsequent expression of endothelial expression of vascular cell adhesion molecule 156; altered structure and function of basement membrane in vitro,57 which are detected in situ in tissues from diabetic animals and humans;56-58 upregulation of proinflammatory cytokines, such as IL-1ß, TNF-, and IL-6; and growth factors such as platelet-derived growth factor.59-62
The irreversible nature of AGEs and the interaction with their receptors63 provides an environment in which tissues and cells are constantly exposed to these products, thereby creating a state of heightened cellular activity. The severity and progression of periodontal disease in diabetes often does not correlate with the classical presentation in a non-systemically challenged patient. The amount of tissue destruction found in patients with diabetes may not correspond to the etiological burden (i.e., bacterial plaque) observed clinically.
The host response during an infectious challenge involves a number of cytokines and hormones of the immune system. These effector molecules serve to modulate the interactions between various cells types involved in the inflammatory process. Inflammation is a complex set of events and involves release of mediators by resident and infiltrating cells. Chronic hyperglycemia with accumulation of AGEs is associated with increased expression of various genes regulated by the transcription factor nuclear factor-B (NF-B).64 Strong evidence has accumulated to indicate that chronic dysregulation of NF-B activation may contribute to many inflammatory diseases, such as periodontal disease.65-68 AGEs and LPS-induced NF-B activation could be responsible for promoting the aberrant transcriptional gene regulation observed in diabetic patients with periodontitis that may be directly related to the accumulation of AGEs intra- and extracellularly.
PERIODONTITIS AND CARDIOVASCULAR OUTCOMES IN PATIENTS WITH DIABETES
Diabetes is a systemic disease with a number of major complications that may adversely affect quality and length of life, particularly as it relates to cardiovascular events and sudden death. Studies to date have reported conflicting associations between oral infection, coronary heart disease, and incident coronary heart disease.69-71 However, there is evidence that dental infection is associated with coronary atherosclerosis and that bacterial DNA has been identified in atherosclerotic plaques,65,66 and other studies have related dental infection to the incidence of coronary events.65,66
The Dental Atherosclerosis Risks in Communities Study is one of the studies providing evidence of a relationship between periodontal infection and presence of subclinical atherosclerosis.67 Also, data available from the Insulin Resistance Atherosclerosis Study has shown that chronic hyperglycemia was positively associated with increased intimal-medial wall thickness (IMT). This study demonstrated an independent association between fasting glucose and individuals with established diabetes and IMT.68
Although studies have reported separately on associations of periodontitis and diabetes and periodontitis and coronary heart disease, the impact of periodontitis on progression of cardiovascular disease in individuals with diabetes has not been extensively investigated. It is believed that infection-mediated upregulation of cytokines and other inflammatory mediators play a central role in this pathological process. The high prevalence of cardiovascular disease and periodontitis in individuals with diabetes may be attributed to an increased inflammatory response leading to atherosclerosis that is usually more extensive and that develops at an earlier age compared to those without diabetes.
ORAL COMPLICATIONS OF DIABETES
Periodontal disease has been reported as the sixth complication of diabetes, along with neuropathy, nephropathy, retinopathy, and micro- and macrovascular diseases.72 Many studies have been published describing the bidirectional interrelationship exhibited by diabetes and periodontal disease. Studies have provided evidence that control of periodontal infection has an impact on improvement of glycemic control evidenced by a decrease in demand for insulin and decreased hemoglobin A1c levels.73-75
In addition to periodontal infection and gingival inflammation, a number of other oral complications have often been reported in patients with diabetes. These include xerostomia, dental caries, candida infection, burning mouth syndrome, lichen planus, and poor wound healing. Proper management of these complications requires that they first must be properly diagnosed. Many of the problems can be properly identified by provision of a comprehensive oral examination at each medical or dental visit.
Periodontal Disease and Gingivitis
The classic presentation of periodontal disease is associated with accumulation of plaque and calculus that harbors bacteria and potent virulence factors, which lead to destruction of periodontal tissues and resorption of alveolar bone around the teeth. Periodontitis is often preceded by various stages of gingival inflammation referred to as gingivitis. Gingivitis is an inflammation of the gums and is the initial and most easily treatable stage of gum disease.
The direct cause of gingivitis is plaque, the soft, sticky, colorless film of bacteria that forms constantly on the teeth and gums. Classic signs and symptoms of gingivitis include red, swollen, tender gums that may bleed upon tooth-brushing. If gingivitis is not treated, it can and often will progress to periodontal disease. The infection then leads to formation of pockets between the teeth and gums signaling breakdown of the periodontal apparatus and bone. Some patients may experience recurring halitosis (bad breath) or a bad taste in the mouth, even if the disease is not advanced. The gum tissue around teeth may also have receded along the root surface, exposing the roots and giving teeth an elongated appearance.
Therapeutic goals for management of periodontal disease and gingivitis in patients with diabetes must involve elimination of infection by removal of plaque and calculus, a decrease in the inflammation response, and maintenance of glycemic control. Management should be accomplished by regular dental cleaning every 6 months by a licensed dental care provider and routine oral self-care (tooth-brushing and flossing) by patients.
Studies have compared the efficacy of different types of toothbrushes (manual, oscillating, or sonic) and have found that the mode of tooth-brushing may affect the amount of plaque retained interproximally.76,77 Several studies have found the oscillating or sonic brushes most effective. The American Dental Association recommends brushing at least twice a day and daily flossing.78,79 Generally, morning and night are convenient brushing times for most people. Toothbrushes should be replaced every 3-4 months. Children’s toothbrushes may need to be replaced more often.
In addition, there are a number of over-the-counter and prescription oral antibacterial rinses that can decrease bacterial load to allow for tissue healing and repair. Listerine and chlorhexidine gluconate (Peridex) have the acceptance and seal of the American Dental Association’s Council on Dental Therapeutics. Listerine involves bacterial cell wall destruction, bacterial enzymatic inhibition, and extraction of bacterial LPS. Chlorhexidine has the ability to bind to hard and soft tissue with slow release.80 Other products that have been shown to have promising antimicrobial effects are mouth rinses and dentifrices containing triclosan.81,82
Based on the amount of progression of periodontal disease, more aggressive therapeutic interventions may be indicated. Therapy may involve surgery, antimicrobials (local or systemic), or a combination of both.
Acute episodes of oral infection in diabetic patients should be addressed immediately. Appropriate antibiotics and pain management should be provided, along with referral to a dentist as soon as possible. The most common antibiotic used for treatment of acute dental infection is amoxicillin; for individuals who are allergic to penicillin, clindamycin is the drug of choice. Because of concerns within the medical and dental communities about the development of antibiotic resistant organisms, the minimum effective dose should be given. The dosage for amoxicillin is 250 mg, three times a day for 7 days, or clindamycin, 300 mg four times a day for 7 days. For patients with uncontrolled diabetes, the dosages may need to be higher and prescribed for longer periods of time to address defective immune and healing responses. Chronic periodontal disease should also be identified, and patients having it should be referred to a dental practitioner for evaluation and treatment.
Xerostomia and Dental Caries
Diabetes can lead to marked dysfunction of the secretory capacity of the salivary glands.83 This process is often associated with salivary gland dysfunction. Xerostomia is qualitative or quantitative reduction or absence of saliva in the mouth. It is a common complication of head and neck radiation, systemic diseases, and medications.
Normal salivary function is mediated by the muscarinic M3 receptor.84-86 Efferent nerve signals mediated by acetylcholine also stimulate salivary glandular epithelial cells and increase salivary secretions.87 Individuals with xerostomia often complain of problems with eating, speaking, swallowing, and wearing dentures. Dry, crumbly foods, such as cereals and crackers, may be particularly difficult to chew and swallow. Denture wearers may have problems with denture retention, denture sores, and the tongue sticking to the palate. Patients with xerostomia often complain of taste disorders (dysgeusia), a painful tongue (glossodynia), and an increased need to drink water, especially at night.
Xerostomia can lead to markedly increased dental caries, parotid gland enlargement, inflammation and fissuring of the lips (cheilitis), inflammation or ulcers of the tongue and buccal mucosa, oral candidiasis, salivary gland infection (sialadenitis), halitosis, and cracking and fissuring of the oral mucosa.88,89 In patients with xerostomia, development of dental caries can be rampant and severe and, if left untreated, can result in infection of the dental pulp and tooth abcess.
The onset of caries requires Streptococci mutans bacteria. These bacteria adhere well to the tooth surface and produce higher amounts of acid from sugars than other bacteria in the mouth. When the proportion of S. mutans in plaque is high (in the range of 2-10%) a patient is at high risk for caries.90-101 The combination of bacteria in the presence of a dry mouth and a source of sugar intake may lead to a high dental caries risk.
Etiology of xerostomia is associated with a noninflammatory, nonneoplastic enlargement of the parotid gland believed to occur in 25% of patients with moderate to severe diabetes and especially in patients with type 1 diabetes and poor metabolic control.102
Diagnosis of xerostomia and caries may be based on evidence obtained from patients’ history or an examination of the oral cavity. Xerostomia would be suspected if a tongue depressor sticks to the buccal mucosa or, in women, if lipstick adheres to the front teeth. The oral mucosa will also be dry and sticky or appear erythematous, which could be the result of an overgrowth of Candida albicans. These can be red or white patches or both, often found on the hard or soft palate or dorsal surface of the tongue. Occasionally, pseudomembranous candidiasis will be present, appearing as removable white plaque on any mucosal surface. There may be little or no pooled saliva in the floor of the mouth, and the tongue may appear dry with decreased numbers of papillae. The saliva may appear stringy, ropy, or foamy. Dental caries may be found at the cervical margin or neck of the teeth (the area where the tooth meets the gum) or the incisal margins (the edges or biting surfaces of teeth). Dry mouth is often exacerbated by activities such as hyperventilation, breathing through the mouth, smoking, or drinking alcohol.
Palliative interventions include saliva substitutes and stimulants. Many products can be purchased directly from the local pharmacy (xerolube, biotene products), while others will require prescription (pilocarpine, cevimeline).
Candidiasis
Oral candida is an infection of the yeast fungus C. albicans. The infection can occur as a side effect of taking medications such as antibiotics, antihistamines, or chemotherapy drugs. Other disorders associated with development of xerostomia include diabetes, drug abuse, malnutrition, immune deficiencies, and old age. Candida is present in the oral cavity of almost half of the population and has been shown be prevalent in people with diabetes as well. Studies have shown a higher prevalence candida in diabetic versus nondiabetic individuals.103 In addition, Geerling et al.104 reported a significantly higher prevalence of candida infection in people with diabetes.
The manifestation of candida can occur in many different forms and include median rhomboid glossitis, atrophic glossitis, denture stomatitis, and angular cheilitis. Candida does not generally become a problem until there is a change in the chemistry of the oral cavity that favors candida over the other micro-organisms present. Contributing factors to infection are salivary dysfunction, a compromised immune system, and salivary hyperglycemia.105,106
Candida infection is also found commonly in denture wearers.107 In the case of infection, the denture should be treated as well as the patient. The denture should be cleaned thoroughly and can be soaked or lined with anti-fungal medication or chlorhexidine. Further, ill-fitting dentures can cause breaks in the mucosal membranes at the corners of the mouth that can act as a nidus for candidal growth.
Treatment of candida infection is fairly straightforward and involves prescribing a therapeutic regimen of antifungals that can be applied locally. Common antifungals used are nystatin, clotrimazole, and fluconazole. Dosage for these medications will depend on the manifestation and extent of the infection and use of pastilles, lozenges, or troches to provide a local as well as systemic effect.
Lichen Planus
Oral lichen planus is a chronic inflammatory disease that causes bilateral white striations, papules, or plaques on the buccal mucosa, tongue, and gingivae. Erythema, erosions, and blisters may or may not be present. The pathogenesis of the disorder is unknown. Studies suggest that lichen planus is a T-cell-mediated autoimmune disease in which cytotoxic CD8+ T-cells trigger apoptosis of the oral epithelial cells.108,109 Microscopically, a lymphocytic infiltrate is described that is composed of T-cells almost exclusively, and many of the T-cells in the epithelium adjacent to the damaged basal keratinocytes are activated CD8+ lymphocytes.
Lichen planus may predispose individuals to cancer and oral C. albicans superinfection.110,111 Fewer than 5% of these patients will develop oral squamous cell carcinoma (SCC).112 Atrophic, erosive, and plaque lesions may pose a greater risk of malignant change, although SCC may arise in the unaffected oral mucosa as well.
The aim of treatment is to eliminate mucosal erythema, ulceration, pain, and sensitivity. This may involve topical or systemic steroid management. The use of steroids in individuals with diabetes may present additional complications, such as antagonism of insulin and subsequent hyperglycemia. Therefore, therapy by the dentist should be done in close consultation with the physician to avoid adverse reactions and drug interactions.
Burning Mouth Syndrome
A combination of factors appears to play a role in this process. Burning mouth syndrome is a chronic, oral pain condition associated with burning sensations of the tongue, lips, and mucosal regions of the mouth. The pathophysiology is mainly idiopathic but can be associated with uncontrolled diabetes, hormone therapy, psychological disorder, neuropathy, xerostomia, and candidiasis.113,114 Generally, there are no detectable lesions associated with the syndrome, which is based solely on patient report of discomfort.
Treatment is targeted at the symptoms and requires attention to glycemic control, which will result in reduction of other complications involved in the process. Medications often used for this condition, benzodiazepines, tricyclic antidepressants, and anticonvulsants, have been shown to be effective therapies. Care must taken prescribing these medications to patients with diabetes because of associated xerostomic effects.
CONCLUSIONS
Maintenance of a healthy dentition for the purpose of asthetics, dietary intake and nutrition, quality of life, and overall general health is the ultimate goal of dental health care. In addition to public awareness and education efforts, much of dental care is focused on effective and efficient preventive and therapeutic management of two major clinical diseases: dental caries and periodontitis.115 While the prevalence of dental caries has declined in many but not all segments of the population, the prevalence of periodontal diseases in individuals with poorly controlled diabetes has been documented.15,116
Many studies conducted during the past decade have focused on a change in approach to studying periodontal infection and its relationship to systemic health and disease. Periodontal diseases are recognized as infectious processes that require bacterial presence and a host response. Risk factors in conjunction with bacteria and the host response can affect the severity of disease, patterns of destruction, and response to therapy.
Many medical conditions, particularly diabetes, predispose patients to development of more severe and progressive forms of periodontal disease.117-119 In an effort to focus attention on the need for better oral health outcomes for patients with diabetes and periodontitis or other oral complications, providers should take several action steps, including:
Ask individuals with diabetes about their oral health, specifically if they have noticed any signs of infection, bad breath, or a bad taste in their mouth or if they have any other symptoms.
Inquire about the last dental and oral health examination.
Remind individuals with diabetes that they need periodic dental and periodontal examinations (every 6 months or more frequently) as recommended by the American Dental Association.
Encourage contact with patients’ dental care provider if they notice signs of infection such as sore, swollen, or bleeding gums; loose teeth; mouth ulcers; or pain.
Perform an oral examination.
Refer all diabetic patients without a dental provider, regardless of oral findings or complaints, to a dentist for preventive care.
Although glycemic control is probably the single most important component in maintenance of good oral health in individuals with diabetes, attention to these action steps will be very helpful toward achieving improved overall oral and systemic health. All of these measures play an important role in maintaining oral health, particularly in diabetic patients.
Many studies have emerged that focus on periodontal infection and systemic disease.120-122 The information from these studies suggests that measures to combat complications of diabetes, especially periodontitis and gingivitis, may be important in reducing additional systemic inflammatory burden, thus potentially preventing diabetes, cardiovascular disease, and other systemic morbidities.
Footnotes
Janet H. Southerland, DDS, MPH, PhD, is an assistant professor in the Department of Dental Ecology and is the chair of Hospital Dentistry, chief of Oral Medicine, and director of the University of North Carolina Hospitals’ Dental Clinic in Chapel Hill. George W. Taylor, DMD, DrPH, is an associate professor in the Department of Cariology, Restorative Sciences and Endodontics at the University of Michigan School of Dentistry in Ann Arbor. Steven Offenbacher DDS, PhD, MMSc, is OraPharma Distinguished Professor of Periodontology and director of the Center for Oral and Systemic Diseases at the University of North Carolina School of Dentistry in Chapel Hill.
REFERENCES
1 Wild S, Roglic G, Green A, Sicree R, King H: Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care27 : 1047-1053,2004[Abstract/Free Full Text]
2 Schmidt MI, Duncan BB, Sharrett AR, Lind-berg G, Savage, PJ, Offenbacher S, Azambuja MI, Tracy RP, Heiss GB: Markers of inflammation and prediction of diabetes mellitus in adults (Atherosclerosis Risk in Communities study): a cohort study. Lancet 353:1649 -1652, 1999[Medline]
3 Slade GD, Offenbacher S, Beck, JD, Heiss G, Pankow JS: Acute-phase inflammatory response to periodontal disease in the US population. J Dent Res 79: 49-57,2000[Abstract/Free Full Text]
4 Brownlee M: The pathological implications of protein glycation. Clin Invest Med 18: 275-281,1995[Medline]
5 Luger A, Schernthaner G, Urbanski A, Luger TA: Cytokine production in patients with newly diagnosed insulin-dependent (type I) diabetes mellitus. Eur J Clin Invest 18:233 -236, 1988[Medline]
6 Nishimura F, Soga Y, Iwamoto Y, Kudo C, Murayama Y: Periodontal disease as part of the insulin resistance syndrome in diabetic patients. J Int Acad Periodontol 7:16 -20, 2005[Medline]
7 Wautier, JL, Schmidt AM: Protein glycation: a firm link to endothelial cell dysfunction. Circ Res 95:233 -238, 2004[Abstract/Free Full Text]
8 Basta G, Lazzerini G, Del Turco S, Ratto GM, Schmidt AM, De Caterina R: At least 2 distinct pathways generating reactive oxygen species mediate vascular cell adhesion molecule-1 induction by advanced glycation end products. Arterioscler Thromb Vasc Biol25 : 1401-1407,2005[Abstract/Free Full Text]
9 Cutler CW, Machen RL, Jotwani R, Iacopino AM: Heightened gingival inflammation and attachment loss in type 2 diabetics with hyperlipidemia. J Periodontol 70:1313 -1321, 1999[Medline]
10 Lamster IB, Lalla E: Periodontal disease and diabetes mellitus: discussion, conclusions, and recommendations. Ann Periodontol6 : 146-149,2001[Medline]
11 Lovegrove JM: Dental plaque revisited: bacteria associated with periodontal disease. J N Z Soc Periodontol 87:7 -21, 2004
12 Lalla E, Lamster IB, Drury S, Fu C, Schmidt AM: Hyperglycemia, glycoxidation and receptor for advanced glycation endproducts: potential mechanisms underlying diabetic complications, including diabetes-associated periodontitis. Periodontol 23:50 -62, 2000
13 Salvi G E, Yalda, B, Collins JG, Jones BH, Smith FW, Arnold RR, Offenbacher S: Inflammatory mediator response as a potential risk marker for periodontal diseases in insulin-dependent diabetes mellitus patients. J Periodontol 68:127 -135, 1997[Medline]
14 Miller LS, Manwell MA, Newbold D, Reding ME, Rasheed A, Blodgett J, Kornman KS: The relationship between reduction in periodontal inflammation and diabetes control: a report of 9 cases. J Periodontol 63:843 -848, 1992[Medline]
15 Tsai C, Hayes C, Taylor GW: Glycemic control of type 2 diabetes and severe periodontal disease in the US adult population. Community Dent Oral Epidemiol 30:182 -192, 2002[Medline]
16 Page RC, Offenbacher S, Schroeder HE, Seymour GJ, Kornman KS: Advances in the pathogenesis of periodontitis: summary of developments, clinical implications and future directions. Periodontol 200014 : 216-248,1997
17 Papapanou PN: Epidemiology of periodontal diseases: an update. J Int Acad Periodontol 1:110 -116, 1999[Medline]
18 Wittebole X, Coyle SM, Kumar A, Goshima M, Lowry SF, Calvano SE: Expression of tumour necrosis factor receptor and Toll-like receptor 2 and 4 on peripheral blood leucocytes of human volunteers after endotoxin challenge: a comparison of flow cytometric light scatter and immunofluorescence gating. Clin Exp Immunol 141:99 -106, 2005[Medline]
19 Kirschning CJ, Wesche H, Merrill Ayres T, Rothe M: Human toll-like receptor 2 confers responsiveness to bacterial lipopolysaccharide. J Exp Med 188:2091 -2097, 1998[Abstract/Free Full Text]
20 Takeda K, Kaisho T, Akira S: Toll-like receptors. Ann Rev Immunol21 : 335-376,2003[Medline]
21 Akira S, Sato S: Toll-like receptors and their signaling mechanisms. Scand J Infect Dis 35: 555-562,2003[Medline]
22 Verma S: C-reactive protein incites atherosclerosis. Can J Cardiol 20 (Suppl. B):29B -31B, 2004
23 Salvi GE, Spets-Happonen S, Singer RE, Offenbacher S: Reconstitution of a hyperinflammatory prostaglandin E2 response to Porphyromonas gingivalis challenge in severe combined immunodeficient mice. J Periodontol 76:16 -21, 2005[Medline]
24 Kurtis B, Develioglu H, Taner IL, Balos K, Tekin IO: IL-6 levels in gingival crevicular fluid (GCF) from patients with non-insulin dependent diabetes mellitus (NIDDM), adult periodontitis and healthy subjects. J Oral Sci 41: 163-167,1999[Medline]
25 Grant-Theule DA: Periodontal disease, diabetes, and immune response: a review of current concepts. J West Soc Periodontol Periodontal Abstr44 : 69-77,1996[Medline]
26 Engebretson SP, Hey-Hadavi J, Ehrhardt FJ, Hsu D, Celenti RS, Grbic JT, Lamster IB: Gingival crevicular fluid levels of interleukin-1beta and glycemic control in patients with chronic periodontitis and type 2 diabetes. J Periodontol 75:1203 -1208, 2004[Medline]
27 Sastrowijoto SH, Abbas F, Abraham-Inpijn L, van der Velden U: Relationship between bleeding/plaque ratio, family history of diabetes mellitus and impaired glucose tolerance. J Clin Periodontol17 : 55-60,1990[Medline]
28 Iacopino AM: Periodontitis and diabetes interrelationships: role of inflammation. Ann Periodontol 6:125 -137, 2001[Medline]
29 Molvig J, Baek L, Christensen P, Manogue KR, Vlassara H, Platz P, Nielsen LS, Svejgaard A, Nerup J: Endotoxin-stimulated human monocyte secretion of interleukin 1, tumour necrosis factor alpha, and prostaglandin E2 shows stable interindividual differences. Scand J Immunol27 : 705-716,1988[Medline]
30 Salvi GE, Beck JD, Offenbacher S: PGE2, IL-1 beta, and TNF-alpha responses in diabetics as modifiers of periodontal disease expression. Ann Periodontol 3:40 -50, 1998[Medline]
31 Kolb H, Mandrup-Poulsen T: An immune origin of type 2 diabetes? Diabetologia 48:1038 -1050, 2005[Medline]
32 Hotamisligi GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM: IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science271 : 665-668,1996[Abstract]
33 Boden G: Interaction between free fatty acids and glucose metabolism. Curr Opin Clin Nutr Metab Care 5:545 -549, 2002[Medline]
34 Hotamisligil GS, Hargill NS, Spiegelman BM: Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 259:87 -91, 1993[Abstract/Free Full Text]
35 Vallance P: Exploring vascular nitric oxide in health and disease: the Goulstonian Lecture 1996. J R Coll Physicians Lond31 : 321-327,1997[Medline]
36 Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD: Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J Clin Invest97 : 2601-2610,1996[Medline]
37 Corbett J, Serup P, Bonner-Weir S, Nielsen JH: Beta-cell ontogeny: growth and death. Diabetologia 40 (Suppl. 3):B27 -B32, 1997
38 Boyko EJ, Leonetti DL, Bergstrom RW, Newell-Morris L, Fujimoto WY: Visceral adiposity, fasting plasma insulin, and blood pressure in Japanese-Americans. Diabetes Care 18: 174-181,1995[Abstract]
39 Bjorklund A, Yaney G, McGarry JD, Weir G: Fatty acids and beta-cell function. Diabetologia 40 (Suppl. 3):B21 -B26, 1997
40 Unger RH: Lipotoxicity in the pathogenesis of obesity-dependent NIDDM: genetic and clinical implications. Diabetes44 : 863-870,1995[Abstract]
41 Wellen KE, Hotamisligil GS: Obesity-induced inflammatory changes in adipose tissue. J Clin Invest 112:1785 -1788, 2003[Medline]
42 Brownlee M: A radical explanation for glucose-induced beta cell dysfunction. J Clin Invest 112:1788 -1790, 2003[Medline]
43 Hotamisligi GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM: Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest 95:2409 -2415, 1995
44 Savage DB, Petersen KF, Shulman GI: Mechanisms of insulin resistance in humans and possible links with inflammation. Hypertension45 : 828-833,2005[Abstract/Free Full Text]
45 Jove M, Planavila A, Laguna JC, Vazquez-Carrera M: Palmitate-induced interleukin 6 production is mediated by protein kinase C and nuclear-factor kappaB activation and leads to glucose transporter 4 down-regulation in skeletal muscle cells. Endocrinology 146:3087 -3095, 2005[Abstract/Free Full Text]
46 Baron AD: Insulin resistance and vascular function. J Diabetes Complications 16:92 -102, 2002[Medline]
47 Rattigan S, Clark MG, Barrett EJ: Hemodynamic actions of insulin in rat skeletal muscle: evidence for capillary recruitment. Diabetes46 : 1381-1388,1997[Abstract]
48 Mattila KJ, Asikainen S, Wolf J, Jousimies-Somer H, Valtonen V, Nieminen M: Age, dental infections, and coronary heart disease. J Dent Res79 : 756-760,2000[Abstract/Free Full Text]
49 Genco R, Offenbacher S, Beck JD: Periodontal disease and cardiovascular disease: epidemiology and possible mechanisms. J Am Dent Assoc133 (Suppl.): 14S-22S,2002[Abstract/Free Full Text]
50 Beck JD, Slade G, Offenbacher S: Oral disease, cardiovascular disease and systemic inflammation.Periodontol 2000 23:110 -120, 2000
51 Yu WK, Li WQ, Li N, Li JS: Influence of acute hyperglycemia in human sepsis on inflammatory cytokine and counterregulatory hormone concentrations. World J Gastroenterol 9:1824 -1827, 2003[Medline]
52 Krogh-Madsen R, Moller K, Dela F, Kronborg G, Jauffred S, Pedersen BK: Effect of hyperglycemia and hyperinsulinemia on the response of IL-6, TNF-alpha, and FFAs to low-dose endotoxemia in humans. Am J Physiol Endocrinol Metab286 : E766-E772,2004[Abstract/Free Full Text]
53 Naguib G, Al-Mashat H, Desta T, Graves DT: Diabetes prolongs the inflammatory response to a bacterial stimulus through cytokine dysregulation. J Invest Dermatol 123:87 -92, 2004[Medline]
54 Zhang L, Zalewski A, Liu Y, Mazurek T, Cowan S, Martin JL, Hofmann SM, Vlassara H, Shi Y: Diabetes-induced oxidative stress and low-grade inflammation in porcine coronary arteries. Circulation108 : 472-478,2003[Abstract/Free Full Text]
55 Vlassara H: The AGE-receptor in the pathogenesis of diabetic complications. Diabetes Metab Res Rev 17:436 -443, 2001[Medline]
56 Hasegawa G, Hunter AJ, Charonis AS: Matrix nonenzymatic glycosylation leads to altered cellular phenotype and intracellular tyrosine phosphorylation. J Biol Chem 270:3278 -3283, 1995[Abstract/Free Full Text]
57 Hong SB, Lee KW, Handa JT, Joo CK: Effect of advanced glycation end products on lens epithelial cells in vitro. Biochem Biophys Res Commun275 : 53-59,2000[Medline]
58 Basta G, Lazzerini G, Del Turco S, Ratto GM, Schmidt AM, De Caterina R: At least 2 distinct pathways generating reactive oxygen species mediate vascular cell adhesion molecule-1 induction by advanced glycation end products. Arterioscler Thromb Vasc Biol25 : 1401-1407,2005
59 Bendayan M: Immunocytochemical detection of advanced glycated end products in rat renal tissue as a function of age and diabetes. Kidney Int54 : 438-447,1998[Medline]
60 Nakayama M, Kawaguchi Y, Yamada K, Hasegawa, T, Takazoe K, Katoh N, Hayakawa H, Osaka N, Yamamoto H, Ogawa A, Kubo H, Shigematsu T, Sakai O, Horiuchi S: Immunohistochemical detection of advanced glycosylation end-products in the peritoneum and its possible pathophysiological role in CAPD. Kidney Int 51: 182-186,1997[Medline]
61 Lalla E, Lamster IB, Stern DM, Schmidt AM: Receptor for advanced glycation end products, inflammation, and accelerated periodontal disease in diabetes: mechanisms and insights into therapeutic modalities. Ann Periodontol6 : 113-118,2001[Medline]
62 Hou FF, Miyata T, Boyce J, Yuan Q, Chertow GM, Kay J, Schmidt AM, Owen WF: Beta(2)-microglobulin modified with advanced glycation end products delays monocyte apoptosis. Kidney Int 59:990 -1002, 2001[Medline]
63 Schmidt AM, Hori O, Brett J, Yan SD, Wautier JL, Stern D: Cellular receptors for advanced glycation end products: implications for induction of oxidant stress and cellular dysfunction in the pathogenesis of vascular lesions. Arterioscler Thromb 14:1521 -1528, 1994[Abstract/Free Full Text]
64 Kirstein M, Brett J, Radoff S, Ogawa S, Stern D, Vlassara H: Advanced protein glycosylation induces transendothelial human monocyte chemotaxis and secretion of platelet-derived growth factor: role in vascular disease of diabetes and aging. Proc Natl Acad Sci U S A87 : 9010-9014,1990[Abstract/Free Full Text]
65 Poligone B, Weaver DJ Jr, Sen P, Baldwin AS Jr: Elevated NF-kappaB activation in nonobese diabetic mouse dendritic cells results in enhanced APC function. J Immunol 168:188 -196, 2002[Abstract/Free Full Text]
66 Liu J, Beller DI: Distinct pathways for NF-kappa B regulation are associated with aberrant macrophage IL-12 production in lupus- and diabetes-prone mouse strains. J Immunol 170:4489 -4496, 2003[Abstract/Free Full Text]
67 Nichols TC, Fischer TH, Deliargyris EN, Baldwin AS Jr: Role of nuclear factor-kappa B (NF-kappa B) in inflammation, periodontitis, and atherogenesis. Ann Periodontol 6:20 -29, 2001[Medline]
68 Beck JD, Eke P, Heiss G, Madianos P, Couper D, Lin D, Moss K, Elter J, Offenbacher S: Periodontal disease and coronary heart disease: a reappraisal of the exposure. Circulation 112:19 -24, 2005[Abstract/Free Full Text]
69 Bierhaus A, Humpert PM, Stern DM, Arnold B, Nawroth PP: Advanced glycation end product receptor-mediated cellular dysfunction. Ann N Y Acad Sci 1043: 676-6802005[Abstract/Free Full Text]
70 Kislinger T, Fu C, Huber B, Qu W, Taguchi A, Du Yan S, Hofmann M, Yan SF, Pischetsrieder M, Stern D, Schmidt AM: N(epsilon)-(carboxymethyl)lysine adducts of proteins are ligands for receptor for advanced glycation end products that activate cell signaling pathways and modulate gene expression. J Biol Chem 274:31740 -31749, 1999[Abstract/Free Full Text]
71 Kumar A, Takada Y, Boriek AM, Aggarwal BB: Nuclear factor-kappa B: its role in health and disease. J Mol Med 82:434 -448, 2004[Medline]
72 Lowe GD: The relationship between infection, inflammation, and cardiovascular disease: an overview. Ann Periodontol 6:1 -8, 2001
73 Danesh J, Appleby P: Persistent infection and vascular disease: a systematic review. Expert Opin Invest Drugs 7:691 -713, 1998
74 Desvarieux M, Demmer RT, Rundek T, Boden-Albala B, Jacobs DR Jr, Sacco RL, Papapanou PN: Periodontal microbiota and carotid intima-media thickness: the Oral Infections and Vascular Disease Epidemiology Study (INVEST). Circulation 111:576 -582, 2005[Abstract/Free Full Text]
75 Fiehn NE, Larsen T, Christiansen N, Holmstrup P, Schroeder TV: Identification of periodontal pathogens in atherosclerotic vessels. J Periodontol76 : 731-736,2005[Medline]
76 Deliargyris EN, Madianos PN, Kadoma W, Marron I, Smith SC Jr, Beck JD, Offenbacher S: Periodontal disease in patients with acute myocardial infarction: prevalence and contribution to elevated C-reactive protein levels. Am Heart J 147: 1005-1009,2004[Medline]
77 Elter JR, Offenbacher S, Toole, JF, Beck JD: Relationship of periodontal disease and edentulism to stroke/TIA. J Dent Res82 : 998-1001,2003[Abstract/Free Full Text]
78 Hujoel PP, Drangsholt M, Spiekerman C, DeRouen TA: Pre-existing cardiovascular disease and periodontitis: a follow-up study. J Dent Res81 : 186-191,2002[Abstract/Free Full Text]
79 Beck JD, Elter JR, Heiss G, Couper D, Mauriello SM, Offenbacher S: Relationship of periodontal disease to carotid artery intima-media wall thickness: the atherosclerosis risk in communities (ARIC) study. Arterioscler Thromb Vasc Biol 21: 1816-1822,2001[Abstract/Free Full Text]
80 Wagenknecht LE, D’Agostino R Jr, Savage PJ, O’Leary DH, Saad MF, Haffner SM: Duration of diabetes and carotid wall thickness: the Insulin Resistance Atherosclerosis Study. Stroke 28:999 -1005, 1997[Abstract/Free Full Text]
81 Loe H: Periodontal disease: the sixth complication of diabetes mellitus. Diabetes Care 16: 329-334,1993[Medline]
82 Grossi SG, Skrepcinski FB, DeCaro T, Robertson DC, Ho AW, Dunford RG, Genco RJ: Treatment of periodontal disease in diabetics reduces glycated hemoglobin. J Periodontol 68:713 -719, 1997[Medline]
83 Taylor GW, Burt BA, Becker MP, Genco RJ, Shlossman M: Glycemic control and alveolar bone loss progression in type 2 diabetes. Ann Periodontol3 : 30-39,1998[Medline]
84 Taylor GW, Burt BA, Becker MP, Genco RJ, Shlossman M, Knowler WC, Pettitt, DJ: Severe periodontitis and risk for poor glycemic control in patients with non-insulin-dependent diabetes mellitus. J Periodontol67 (Suppl. 10): 1085-1093,1996[Medline]
85 Sjogren K, Lundberg AB, Birkhed D, Dudgeon DJ, Johnson MR: Interproximal plaque mass and fluoride retention after brushing and flossing: a comparative study of powered tooth-brushing, manual toothbrushing and flossing. Oral Health Prev Dent 2: 119-124,2004[Medline]
86 Van der WeijdenGA, Timmerman MF, Piscaer M, IJzerman Y, Van der Velden UL: Plaque removal by professional electric toothbrushing compared with professional polishing. J Clin Periodontol31 : 903-907,2004[Medline]
87 Sharma NC, Galustians HJ, Qaqish J, Cugini M, Warren PR: The effect of two power tooth-brushes on calculus and stain formation. Am J Dent 15: 71-76,2002[Medline]
88 Bauroth K, Charles CH, Mankodi SM, Simmons K, Zhao Q, Kumar LD: The efficacy of an essential oil antiseptic mouthrinse vs. dental floss in controlling interproximal gingivitis: a comparative study. J Am Dent Assoc134 : 359-365,2003[Abstract/Free Full Text]
89 Mandel ID: Antimicrobial mouthrinses: overview and update. J Am Dent Assoc 125 (Suppl. 2):2S -10S, 1994[Medline]
90 Wicht MJ, Haak R, Kneist S, Noack MJ: A triclosan-containing compomer reduces Lactobacillus spp. predominant in advanced carious lesions. Dent Mater 21:831 -836, 2005[Medline]
91 Brading MG, Cromwell VJ, Jones NM, Baldeck JD, Marquis RE: Anti-microbial efficacy and mode of action studies on a new zinc/Triclosan formulation. Int Dent J 53 (Suppl. 1):363 -370, 2003[Medline]
92 Mata AD, Marques D, Rocha S, Francisco H, Santos C, Mesquita MF, Singh J: Effects of diabetes mellitus on salivary secretion and its composition in the human. Mol Cell Biochem 261:137 -142, 2004[Medline]
93 Nakamura T, Matsui M, Uchida K, Futatsugi A, Kusakawa S, Matsumoto N, Nakamura K, Manabe T, Taketo MM, Mikoshiba K: M(3) muscarinic acetylcholine receptor plays a critical role in parasympathetic control of salivation in mice. J Physiol 558:561 -575, 2004[Abstract/Free Full Text]
94 Tobin G, Giglio D, Gotrick B: Studies of muscarinic receptor subtypes in salivary gland function in anaesthetized rats. Auton Neurosci100 : 1-9,2002[Medline]
95 Matsui M, Motomura D, Karasawa H, Fujikawa T, Jiang J, Komiya Y, Takahashi S, Taketo MM: Multiple functional defects in peripheral autonomic organs in mice lacking muscarinic acetylcholine receptor gene for the M3 subtype. Proc Natl Acad Sci U S A 97:9579 -9584, 2000[Abstract/Free Full Text]
96 Gautam D, Heard TS, Cui Y, Miller G, Bloodworth L, Wess J: Cholinergic stimulation of salivary secretion studied with M1 and M3 muscarinic receptor single- and double-knockout mice. Mol Pharmacol66 : 260-267,2004[Abstract/Free Full Text]
97 Atkinson JC, Grisius M, Massey W: Salivary hypofunction and xerostomia: diagnosis and treatment. Dent Clin North Am49 : 309-326,2005[Medline]
98 Greenspan D: Xerostomia: diagnosis and management. Oncology (Williston Park) 10 (Suppl. 3):7 -11, 1996
99 Levato CM: Caries management: a new paradigm. Compend Contin Educ Dent26 (Suppl. 6A): 448-454,2005
100 Petersson GH, Fure S, Twetman S, Bratthall D: Comparing caries risk factors and risk profiles between children and elderly. Swed Dent J28 : 119-128,2004[Medline]
101 Banas JA: Virulence properties of Streptococcus mutans. Front Biosci 9:1267 -1277, 2004[Medline]
102 Russotto SB: Asymptomatic parotid gland enlargement in diabetes mellitus. Oral Surg Oral Med Oral Pathol 52:594 -598, 1981[Medline]
103 Fisher BM, Lamey PJ, Samaranayake LP, MacFarlane TW, Frier BM: Carriage of Candida species in the oral cavity in diabetic patients: relationship to glycaemic control. J Oral Pathol 16:282 -284, 1987[Medline]
104 Geerlings SE, Hoepelman AI: Immune dysfunction in patients with diabetes mellitus (DM). FEMS Immunol Med Microbiol 26:259 -265, 1999[Medline]
105 Akpan A, Morgan R: Oral candidiasis. Postgrad Med J78 : 455-459,2002[Abstract/Free Full Text]
106 Rossie K, Guggenheimer J: Oral candidiasis: clinical manifestations, diagnosis, and treatment. Pract Periodontics Aesthet Dent9 : 635-641,1997[Medline]
107 Webb BC, Thomas CJ, Willcox MD, Harty DW, Knox KW: Candida-associated denture stomatitis: aetiology and management: a review. Part 3. Treatment of oral candidosis. Aust Dent J 43:244 -249, 1998[Medline]
108 Lodi G, Scully C, Carrozzo M, Griffiths M, Sugerman PB, Thongprasom K: Current controversies in oral lichen planus: report of an international consensus meeting. Part 2. Clinical management and malignant transformation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 100:164 -178, 2005[Medline]
109 Thornhill MH: Immune mechanisms in oral lichen planus. Acta Odontol Scand 59:174 -177, 2001[Medline]
110 Gandolfo S, Richiardi L, Carrozzo M, Broccoletti R, Carbone M, Pagano M, Vestita C, Rosso S, Merletti F: Risk of oral squamous cell carcinoma in 402 patients with oral lichen planus: a follow-up study in an Italian population. Oral Oncol 40: 77-83,2004[Medline]
111 Huber MA: Oral lichen planus. Quintessence Int35 : 731-752.2004[Medline]
112 Sugerman PB, Savage NW: Oral lichen planus: causes, diagnosis and management. Aust Dent J 47:290 -297, 2002[Medline]
113 Rhodus NL, Carlson CR, Miller CS: Burning mouth (syndrome) disorder. Quintessence Int 34: 587-593,2003[Medline]
114 Scala A, Checchi L, Montevecchi M, Marini I, Giamberardino MA: Update on burning mouth syndrome: overview and patient management. Crit Rev Oral Biol Med 14: 275-291,2003[Abstract/Free Full Text]
115 Greenstein G: Changing periodontal concepts: treatment considerations. Compend Contin Educ Dent 26:81 -22, 84-86, 88 passim, 2005
116 Taylor GW, Manz MC, Borgnakke WS: Diabetes, periodontal diseases, dental caries, and tooth loss: a review of the literature. Compend Contin Educ Dent 25: 179-184,186 -188, 190, 2004
117 Campus G, Salem A, Uzzau S, Baldoni E, Tonolo G: Diabetes and periodontal disease: a case-control study. J Periodontol 76:418 -425, 2005[Medline]
118 Guzman S, Karima M, Wang HY, Van Dyke TE: Association between interleukin-1 genotype and periodontal disease in a diabetic population. J Periodontol 74:1183 -1190, 2003[Medline]
119 Mattson JS, Cerutis DR: Diabetes mellitus: a review of the literature and dental implications. Compend Contin Educ Dent22 : 757-760, 762, 764 passim, 2001
120 Nishimura F, Soga Y, Iwamoto Y, Kudo C, Murayama Y: Periodontal disease as part of the insulin resistance syndrome in diabetic patients. J Int Acad Periodontol 7:16 -20, 2005
121 Mealey BL: Diabetes and periodontal disease: two sides of a coin. Compend Contin Educ Dent 21:943 -946, 948, 950, passim, 2000
122 Teng YT, Taylor GW, Scannapieco F, Kinane DF, Curtis M, Beck JD, Kogon S: Periodontal health and systemic disorders. J Can Dent Assoc68 : 188-192,2002
http://clinical.diabetesjournals.org/cgi/reprint/23/4/171
Robert Genco, Chairman of the Oral Biology Department at SUNYAB, shows in studies that treating periodontal infection may reduce a diabetic’s blood sugar. Grossi et al. 1997: Treatment of periodontal disease in diabetics reduces glycated hemoglobin. J. Periodontal 68(8): 713-719.